Here is the "Electricity - Basic Navy Training Courses"
(NAVPERS 10622) in its entirety. It should provide one of the Internet's
best resources for people seeking a basic electricity course - complete with examples
worked out. See
copyright. See
Table of
Contents. • U.S. Government Printing Office; 1945 - 618779
Chapter 5
EMF
What
IT IS
(* VERY IMPORTANT note: current flow here is defined as from negative
to positive, which is
opposite of today's convention. Modern convention of positive-to-negative
current flow, with negative-to-positive electron flow requires a
"right-hand
rule." See Right-Hand
Rule page on RF Cafe).
You have learned that potential difference causes electrons to flow through
a conductor. But-you'll have to know about another "electron-mover." Because
it is an "electron-moving-force," scientists have named it ELECTROMOTIVE
FORCE (EMF). Mechanical force is usually measured in pounds, but EMF is measured
in VOLTS. Just as pounds of force make water flow through a pipe, so EMF makes current
flow through a conductor.
The three terms - POTENTIAL DIFFERENCE, ELECTROMOTIVE FORCE and VOLTAGE - are
often used interchangeably. You will hear electricians say, "VOLTAGE of the
generator"; or "EMF of the generator" and "VOLTAGE of the circuit"
or "POTENTIAL of the circuit." However, note the technical distinctions
in these terms. To be ABSOLUTELY CORRECT, EMF should be applied only to the force
produced by a generator or battery. Example - "The EMF of the generator is
120 volts." POTENTIAL or POTENTIAL DIFFERENCE applies to a total circuit or
a part of a circuit. Example - "The potential difference or drop (p.d.) is
63 volts." The term, VOLTAGE, applies to the number of volts concerned in either
case. Example--"The lamp has a voltage of 120 volts."
Electricians, and sometimes books, confuse these three terms. Don't let it bother
you. Just REMEMBER that to all practical purposes, EMF, potential, and voltage mean
the same thing-the FORCE THAT MAKES current FLOW.
WHERE IT COMES FROM
When a scientist studies a moving automobile, this is what he sees –
First, a gasoline tank full of chemical energy. Second, an engine which burns
this gasoline and uses the heat energy released to turn a crankshaft. Third, the
mechanical energy of the turning crank-shaft transferred as a force 'on the wheels
which move the automobile.
The automobile engine, then, is simply a machine which converts chemical energy
into mechanical energy. A steam engine is similar. It takes the chemical and heat
energy out of coal or oil and converts it to mechanical energy in the form of force
on moving parts. No matter what kind of engine or motor you select, you will find
that each of them converts one kind of energy into another kind and then transfers
the energy to a force which does the work.
Electrical energy - EMF - can be produced by the conversion of four kinds of
energy - mechanical energy, chemical energy, frictional energy, and heat energy.
To change these forms of energy into EMF requires "engines" just as the
automobile requires an engine to convert chemical energy into mechanical energy.
These electrical "engines" are the batteries and generators of your circuits.
EMF FROM MECHANICAL ENERGY - the GENERATOR
The GENERATOR is the "engine" which converts mechanical energy into
electrical energy. It is the most economical and by far the most common source of
EMF.
Generators consist of two parts - a stationary FRAME and a rotating ARMATURE.
The armature is connected to a source of mechanical energy, called a PRIME MOVER
- usually a turbine, or a gasoline or diesel engine. The prime mover furnishes the
energy which rotates the armature. Then the armature, by a process to be explained
later, converts the mechanical energy to electrical energy. Figure 25 is a representation
of a ship's power plant. notice the different forms of energy as each machine makes
a conversion. Finally, at the generator, the energy is in the form of electricity
ready to be sent out on the ship's wires to be used to run motors, light lights,
power radios, and heat galley stoves.
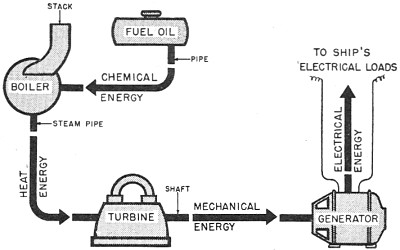
Figure 25. - Ship's power - oil to electricity.
The exact mechanism by which a generator converts mechanical energy to electrical
energy is very complex. The generator is treated in detail in a later chapter of
this book. The important idea to remember here is that GENERATORS FURNISH A CONTINUOUS
EMF TO A CIRCUIT.
EMF FROM CHEMICAL ENERGY - the BATTERY
Much energy is stored by nature in chemical compounds (combinations of elements).
Coal, wood, and oil have tremendous stores of energy which are released as heat
when these compounds are burned. Oxygen and hydrogen have so much energy that they
explode when they combine. The electrician is interested in these - substances only
because he can get some of this energy as EMF.
Releasing electrical energy from chemical energy is surprisingly simple. If
two dissimilar metals - copper and zinc, for example - are placed in certain chemical
solutions, an EMF results. This is the principle employed in all CELLS and BATTERIES
- a battery is simply TWO OR MORE cells connected together.
HOW A CELL WORKS
When two or more atoms of different elements combine, they produce a molecule
of a COMPOUND. For example, atoms of the elements carbon and oxygen combine to form
molecules of carbon dioxide. Carbon dioxide is a COMPOUND, consisting of a combined
form of the elements carbon and oxygen.
When a compound dissolves in certain substances - notably water - it breaks
up into CHARGED PARTICLES. These charged particles are called IONS. Ions are not
the same as atoms-ions are charged and atoms are not. You will remember that atoms
contain an equal number of protons and electrons and therefore are neutral. But
an ion of a dissolved compound either loses or gains one or more electrons. If you
were to dissolve one molecule of sodium chloride - common table salt - in water,
it would split into a sodium ion and a chloride ion. But the chloride ion holds
on to one of the sodium ion's electrons. This gives the chloride ion a negative
charge and the sodium ion a positive charge. It has been proved experimentally that
a solution containing ions will conduct an electric current. The ions seem to "ferry"
the current through the solution. This should explain to you why salt water is so
likely to produce short circuits aboard ships. Because compounds that form ions
in solution will conduct electric currents, they are called ELECTROLYTES.
ELECTRON FLOW
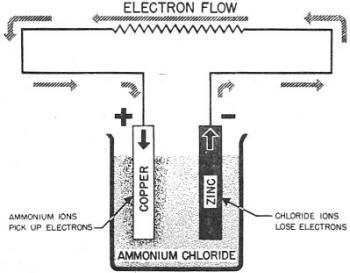
Figure 26. - Voltaic cell.*
All this leads you to an understanding of the workings of a cell. If any two
dissimilar metal plates are placed in an electrolyte, the ions will develop an EMF
at the plates. If the dissimilar plates are then connected by means of a conductor
outside the solution, the EMF will force a current through this conductor. This
is called a VOLTAIC cell (after Volta, an early Italian experimenter).
Here's an example of how the Voltaic cell works. Immerse a copper plate and
a zinc plate in a solution of ammonium chloride, as in figure 26. The positive ammonium
ions pick up electrons from the copper plate. This reduces the number of electrons
on the COPPER PLATE and gives it a POSITIVE charge. The chloride ions give up their
excess electrons to the zinc plate. This gives the ZINC plate, an excess of electrons
and, therefore, a NEGATIVE charge. notice that you have two charged plates - one
positive and one negative. If a wire is connected to the two plates, the potential
difference between its two ends will cause a current to flow.
A number of changes occur in both the plates and electrolyte as the current
flows. The most important change occurs at the zinc plate. Electrons are constantly
being lost by the zinc plate as the current flows, and the zinc atom changes to
a zinc ion - which dissolves in the solution. In short, the zinc is EATEN A WAY
by the action. When the zinc is completely ionized (dissolved) - the cell's EMF
ceases. Because the action of the cell uses up a primary part, the cell is called
a PRIMARY cell. Such a cell cannot be recharged.
The most common primary cell is one you have seen many times - the "dry
cell." Figure 27 shows a cross section view of a dry cell. The two plates,
called ELECTRODES, are zinc and carbon. notice how the zinc electrode is shaped
to form a cylindrical can. Thus, the electrode serves as the cell case. The electrolyte
is ammonium chloride dissolved in water and mixed with paste. The paste is merely
to prevent the electrolyte from spilling.
The chloride ions lose electrons to the zinc plate, giving it a negative charge.
The ammonium ions pick up electrons from the carbon rod giving this electrode a
positive charge. In this type of cell about 1.5 volts of EMF are developed. Once
the outside circuit is completed, the zinc begins to dissolve. Since this is a primary
cell, the action ceases when all the zinc is used up. Usually the paste electrolyte
will leak from a "dead" cell because the zinc container is eaten away.
To avoid messy leakage, dry cells should be removed from flashlights, lanterns,
and radios when the gear is stowed or the batteries are worn out.
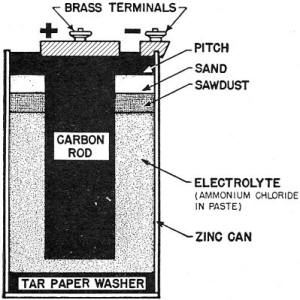
Figure 27. - Cross section of a dry cell.
There are many kinds of primary cells - differing from each other in the materials
used for electrodes and electrolytes. The amount of EMF produced by each depends
on the materials composing the electrodes and electrolyte.
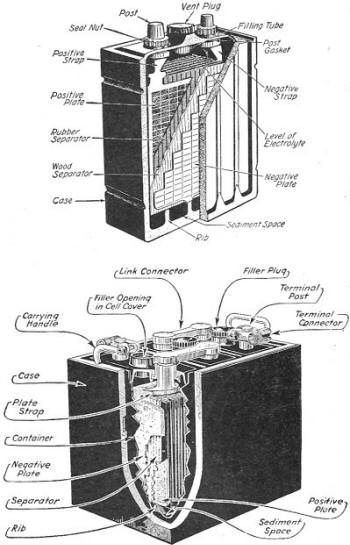
Figure 28. - Lead-acid storage battery.
SECONDARY CELLS
Primary cells are useful for only a short time. Their chemical energy is used
up and they must be discarded. SECONDARY cells also lose their chemical energy,
BUT, they can be restored by passing an electric current through them. The lead
storage cell is a common example of the secondary cell. It is used in battery form
(two or more cells connected together) in automobiles, motor launches and submarines.
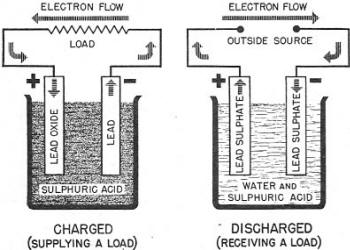
Figure 29. - Charging and discharging the lead storage
battery.*
The lead storage cell has electrodes of lead and lead oxide immersed in a solution
of sulphuric acid. The complete construction is shown in figure 28. During use,
both plates are changed to lead sulphate, and much of the sulphuric acid is converted
to water. These changes mean that the chemical energy of the cell has been converted
to electrical energy. The cell is DISCHARGED. If a current from another source is
passed through this cell IN the OPPOSITE DIRECTION TO THAT OF DISCHARGE, the CHEMICAL
ENERGY IS RESTORED. That is, the plates again become lead and lead oxide and the
water is changed back to sulphuric acid. The cell is now CHARGED and ready to deliver
an EMF. Figure 29 shows the difference between a charged and discharged cell. With
proper care, a secondary cell can be charged and discharged many many times.
EMF FROM FRICTION
Friction between two unlike substances results in a potential difference between
those substances. This potential difference is an EMF which will move electrons
toward the lower potential. You are familiar with this kind of EMF production-it
is the STATIC CHARGE. Although, this was the earliest discovered method of producing
an EMF, there is relatively little practical use for static electricity. Most of
it is wasted as static discharges.
EMF FROM HEAT
When two unlike metals, such as platinum and rhodium, are bound together and
heated, they produce an EMF. This arrangement of two metals is called a THERMOCOUPLE
The strength of the EMF produced by any thermo-couple is proportional to the temperature.
Thermo-couples are used in steel furnaces, boiler flues, stacks, and molten metals
to measure extremely high temperatures. notice in figure 30 that the two wires are
twisted together at .one end and then welded. This is the end to be heated. The
balance of the wires are insulated by porcelain beads to prevent a short circuit.
A very sensitive voltmeter is used to measure the EMF produced by the thermo-couple.
In the example used-a platinum and rhodium thermocouple-a temperature of 920 °F.
will produce 0.003672 volt but a temperature of 1980 °F. will produce 0.010534 volt.
notice that increasing the temperature increases the voltage of the EMF. When a
thermo-couple is used to measure temperature, the voltmeter is calibrated IN DEGREES
INSTEAD OF IN VOLTS. Thus the voltmeter reads directly the temperature of the thermo-couple.
The thermocouple, used to measure temperature, is the only practical use made
of EMF produced by heat.
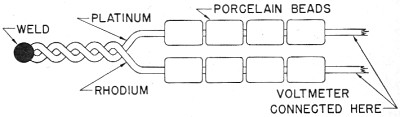
Figure 30. - Thermocouple construction.
Chapter 5 Quiz
(click here)
|















