|
March 23, 1942 Life
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early
technology. See articles from Life magazine,
published 1883-1972. All copyrights hereby acknowledged.
|
In 1942 and throughout the War Years,
Life magazine (and many others) ran many articles promoting industries,
services, organizations, and individuals who contributed toward our ultimate victory.
Of course no one knew for certain that we would prevail in the end, but if it hadn't
turned out that way, it wouldn't have been for lack of effort and sacrifice. Part
of the objective was to inform the populace about how the country was pooling its
resources - physical, labor, and mental - to defeat the Axis Powers that sought
to takeover the world. This particular issue of Life focused on the chemical
industry, with the raw materials and processes used to produce needed products both
for fuel and for the base components of other finished goods. Sulphur, potassium,
and coal mining and processing, along with petroleum, common table salt, and air
and water were some of the most fundamental ingredients of every other item needed
to aid the effort. Ever hear of
Ameriopl rubber?
Industrial Chemistry - It Meets Demands of War

This mountain of coal at a DuPont plant is not fuel but a storehouse
of 150,000 different chemical compounds.
To keep the U. S. forces fighting, industrial chemistry must for the duration
produce 5 lb. of explosives for every soldier every day. The filling of this order
has brought none of the agony of retooling and conversion that has beset other items
of war production. To fractionate toluol from coal for TNT requires only an adjustment
of pressure and temperature controls on the towers that distill the raw materials
for dyes, drugs and plastics. The nitric acid that turns cotton into smokeless powder
is the same nitric acid that turns cotton into plastics and paint.
But the biggest job of industrial chemistry is now, as always, to supply other
industries with the materials it has taught them to use. Without sulphuric acid,
compounded from gleaming plateaus of brimstone pumped up from beneath Gulf Coast
swamplands (see opposite), ten major U. S. industries would find their operations
in confusion. If to sulphuric acid are added the acids, bases, salts and solvents
that chemistry assembles out of water, air, salt, limestone, potash, coal and petroleum,
there is involved the whole fabric of technology by which modern civilization lives
at peace and fights its wars.


Sulphur
Brilliant golden sulphur, the brimstone
of Scriptures, is the quintessential element of all modern industry. As sulphuric
acid, it is crucial in the production of fertilizers, explosives, paint, in the
refining of metals and petroleum, and in almost all chemical processing. The consumption
of sulphuric acid is a reliable and sensitive index of all industrial production.
Biggest single source is the deposit on the Gulf of Mexico in Louisiana and Texas,
mined by a unique process. Through a system of pipes reaching down under 1,500 ft.
of quicksand and cap rock, super-heated water is forced into the sulphur-bearing
stratum. The sulphur, melted, is pumped to the surface and, as shown here at the
works of the Freeport Sulphur Co., spewed out into deep bins. When it has cooled
and hardened, the bins are taken down, leaving rectangular mountains of sulphur,
ready to be blasted down for shipment.

Potash
Potassium, called potash in its useful
compounds, and nitrogen (below) are two major props of modern agriculture provided
by the chemical industry. Both are essential to life. and both tend to disappear
from cultivated land. Of the 500,000 tons of potash to be spread on U. S. soil in
1942, not one ton will be imported, in contrast to 1913 when Germany supplied almost
all our potash. Potassium, sodium and calcium (below) are the most active metals,
ranking just ahead of magnesium and aluminum and too explosive to be used as pure
metals. Like sodium. potassium is found in compounds with chlorine and, like most
of our common salt, is mined from the beds of ancient seas. Shown here is the rosy-pink
potash ore mined by the Potash Company of America, in the Permian Sea deposit, 1,000
ft. below New Mexico's soil. Industrially, most potash goes into matches, a little
into percussion explosives.
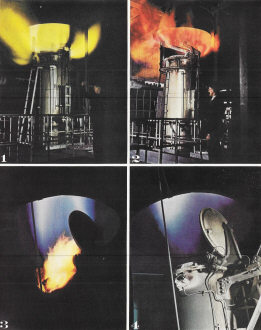
Air & Water
This pyrotechnic display
is a by-product of one of the most vital peace and wartime chemical processes, the
fixation of atmospheric nitrogen. Because it tends constantly to return to its free
state as a gas, nitrogen must be restored in synthetic fertilizers to the soil,
where it is essential to life. In war, nitrogen is the ingredient that makes trinitrocellulose
and trinitrotoluene (TNT), our chief explosives. This gas generator in a du Pont
plant is performing the first step in the fixation of nitrogen with hydrogen in
the form of ammonia. Into a coke-filled furnace below are shot alternate blasts
of air and steam. The oxygen content of both blasts goes into combustion, leaving
nitrogen and hydrogen to be later clamped together by heat and pressure. Excess
gases of the air blast flare in yellow and red flame (top); excess water gas (below)
burns in vivid blue.

Coal
The sooty smog that blankets soft-coal-burning
industrial cities is made of the same chemicals that go into the collection of objects
shown above. When roasted in a by-product coke oven, bituminous coal yields gases,
oils and tars that are even richer than petroleum in hydrocarbon compounds. Black
coal tar is. first of all, the exclusive source of our full spectrum of dyes (in
retorts and swatches). Closely related to these dyes are the coal-tar drugs, including
the miraculous five sulfa compounds (brown) bottles). In the field of plastics,
coal tar is a universal raw material, providing bonding plastics for plywood and
impregnated cloth gears and miners' helmets (left); molding plastics for, among
other things, Bakelite telephones; and surfacing plastics for automobile enamel
(green bottle, right); and finally nylon stockings. Pure carbon from coal pitch
makes arc-light electrodes (left). Coke ovens also produce fuel gas (right).
By the tragic irony that links the release of man's creative energies to his
impulse for destruction, the history of chemistry is the history of war. Modern
industrial chemistry dates from the British blockade of Napoleon's Europe in the
first years of the 19th Century. To supply gunpowder for the Grand Army, French
chemists treated salt with sulphuric acid, and therewith launched three fundamental
chemical commodities, chlorine for bleaching, soda ash for the manufacture of soap
and glass, and sulphuric acid for all its infinity of uses. To World War I must
be credited the next great advance in industrial chemistry. It is a fact that Germany
went to war as soon as its supply of nitrogen for explosives was assured by German
chemistry's invention of a process for artificial fixation of nitrogen from the
air. That process, now again diverted to war production, stands between man and
starvation. Via synthetic fertilizers it restores nitrogen to the soil whence it
goes into proteins, the stuff of life.
To the U. S. in the third year of World War I, Germany twice sent a submarine
loaded with the most precious cargo it could carry - drugs and dyes distilled from
coal tar. These had simply disappeared from consumption in the U. S., where coal
coked for the steel industry spewed its riches through the roof ports of beehive
coke ovens. The same crisis held throughout the U. S. chemical economy, which produced
no potash and only part of our needs of such vital elements as chlorine and nitrogen.
Fortunately the U. S. lacked neither chemical brains nor resources. By the war's
end, because we needed it to fight, we had an almost self-sufficient chemical industry.
Today its independence of foreign sources is complete.
In 1940 U. S. industrial chemistry was supplying its heavy chemicals to other
industries in tonnages far in excess of the production peak of World War I. This
is an index not only of the expansion of U. S. industry but of its mastery of chemical
technology. It is no longer possible to define the limits of the chemical industry.
The metals industry, with its alloys, and the petroleum industry, with its synthetic
fuels, have notably ceased to be mere refiners of natural materials and have become
chemical creators of new materials. Wood products are being revolutionized by coal-tar
resins that bind plywood into sheets stronger than steel. To textiles, chemistry
has brought synthetic fibers that compete with nature's whole line of silk, cotton
and wool. From four of its most inexhaustible raw materials, air, water, limestone
and coal, industrial chemistry has compounded its own industry, producing in sheet
and molded plastics materials that have no precedent in nature.
Already in World War II, U. S. industrial chemistry has begun to project the
lines of its future progress. Its first major triumph is magnesium, heretofore an
incendiary curiosity, now to become, in light, high-strength alloys, the most efficient
aircraft metal. Because it must provide more powerful aviation fuels and replace
lost sources of rubber, it is distilling and cracking an ever longer list of the
rich hydrocarbons locked in coal and oil.
On the following color pages are shown the raw materials of industrial chemistry.
Next, in color (pp. 74-75), the principles of the science of chemistry are diagramed.
This is followed by demonstrations of basic industrial processes which, as any high-school
student can see, are his own experiments many times magnified.
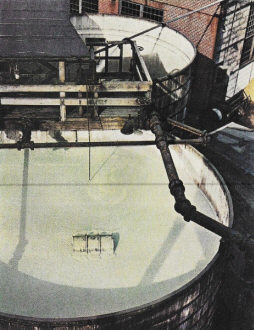
Salt
Common table salt, here pumped in a
brine from a natural well into purifying tanks at the Westvaco plant, is composed
of two violent, poisonous elements, chlorine and sodium. Separated from each other
by electrolysis, each has its own important use. Chlorine is not only a battlefield
gas; it is a powerful germicide and bleaching agent. Sodium compounds into household
lye and is essential to glass, paper and textile manufacturing.
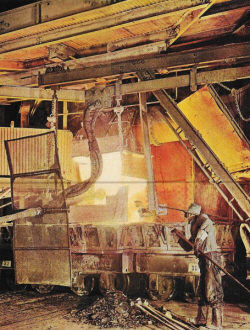
Limestone
A compound of the element calcium,
limestone has long done duty in cement, mortar, plaster and quicklime. Here, in
a Union Carbide furnace. limestone is roasted with coke to incandescence to produce
calcium carbide. Mixed with water, calcium carbide yields the hydrocarbon acetylene
gas that burns in welding torches and is an ingredient in nylon and a synthetic
rubber.

Petroleum
Central object in this display
is a jug of crude petroleum. With it is shown a sampling of the products which chemistry
has begun to distill, crack and synthesize from its green-black and evil-smelling
collection of hydrocarbon compounds. Most spectacular is the Plexiglas bomber nose
housing the display, which is made from a resin compounded from the highly reactive
ethylene fraction of crude. Far more important, however, are the new synthetic 80-
and 100-octane motor fuels, dyed red and blue respectively. It is 100 octane fuel
that gives U. S. warplanes their supreme range and altitude. The jugs of fuel stand
on a yellow sheet of crude Ameripol rubber. whose crucial ingredient is the butadiene
fraction. When the higher fractions and the lower waxes and oils have been distilled
off, there remains solid black "petroleum coke," a cheap industrial fuel.
Posted April 14, 2022
|


















