|
March 23, 1942 Life
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early
technology. See articles from Life magazine,
published 1883-1972. All copyrights hereby acknowledged.
|
In the early days of America's
official involvement in World War II (we were unofficially involved in supplying
equipment and strategy much earlier), much effort was expended in educating the
public on the implements and tactics of war. Doing so help engaged citizens and
give them a sense of involvement. Motivating young men (primarily) to volunteer
to go far from home to fight an enemy in places most had never heard of before
was a tall order. Sure, a forced conscription was implemented (the country's
first
peacetime draft beginning September 16, 1940), but patriotic volunteers are
generally preferred for leadership and long-term commitment to achieving
victory. That's not to say draftees were not likely to turn out being leaders
and career men. Interestingly, so many American men were volunteering for duty
that a presidential order was issued in December of 1942 banning volunteer
service; the government would be the sole determiner of who would be in the
service. ...but I digress. This "Nitrogen Makes High Explosives for Modern War"
article appeared in the March 23, 1942 issue of Life magazine.
Nitrogen Makes High Explosives for Modern War

High-explosive 75-mm. round is loaded with seven charges of six
different kinds of nitrogen explosives.
Modern war is the product of the chemical reaction of explosion. In this process,
a solid of comparatively small volume is translated in a fraction of a second into
gases occupying a relatively huge volume. The 75-mm. shell and case diagramed above
is a reaction chamber for a train of six different explosives. Their one common
ingredient, and the one that makes them all explosive, is nitrogen, which is thus,
far more than iron, the element that fights wars.
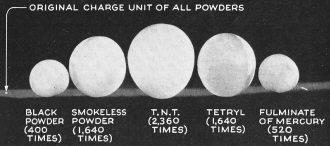
Expansion Ratio, from solid to gas, of five
nitrogen explosives in a 75-mm. round is indicated by balloons, with the dot at
the left representing size of solid charge of each. Compounding of smokeless powder
or nitrocellulose is demonstrated below. Tetryl and TNT are both coal-tar explosives.
Variety of explosives insures safety and precision.
Nitrogen, chief constituent of our atmosphere, makes its compounds explosive
because it constantly seeks its state as a free gas. For this same reason, natural
deposits of nitrogen compounds are rare. Until 1900, warfare was limited by the
supply of naturally fixed nitrogen. Artificial nitrogen fixation (page 71) has since
made war on its present scale possible. Modern war is given extra violence by toluol,
made from coal tar and petroleum (bottles of clear fluid, opposite), which compounds
with nitrogen into TNT.

(L) Wad of cotton burns slowly -- (M) Cotton is nitrated and
becomes nitrocellulose -- (R) Nitrocellulose is explosive
Posted February 21, 2022
|