July 1959 Popular Electronics
 Table
of Contents Table
of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
No, the electrolysis and
corrosion of boat propellers is not really in line with the theme of RF Cafe; however,
it presents the same sort of problems that grounding and anchoring systems for radio
antennas and equipment shacks have. If you bury a piece of metal in the Earth, it
will, over time, magically disappear. Much effort has been expended on the part
of both amateurs and professionals to mitigate the anodic action that occurs when
dissimilar conductors come into intimate contact because each metal - be it a base
or an alloy - has an electric potential relative to other metals. What happens when
there is a difference of potentials and a conduction path is present? Yep, current
flows. Through that action, material is physically transferred from the more positive
metal to the less positive metal. A relatively simple solution was discovered more
than a hundred years ago - a sacrificial element whose only purpose is to supply
the electrons, and hence material loss, in lieu of the important structure. It really
works. There was an article in the ARRL's
QST magazine
that did an excellent job covering this topic.
Note: I was able to exploit the electrolysis process for good to electroplate
some hardware on my 1941
Crosley 03CB console radio.
Electrolysis and Corrosion
 How to save your boat from the ravages of electrical
corrosion How to save your boat from the ravages of electrical
corrosion
By Elbert Robberson
To a jet pilot, it's a flameout. To a parkway pilot, it's a flat tire in the
Holland Tunnel. For the owner of a boat, it's electrolysis - a boating problem ever
since metal parts have found their way aboard.
Talk to old-time boatmen, dealers, or shipyard mechanics, and you'll probably
hear plenty about this sea-water scourge ... stories of boat and engine parts falling
off, propellers turned to lace, boats sinking. It's true that these things can happen
because of electrolysis, but no conscientious boatman need suffer - because electrolysis
is easily prevented. First, however, let's find out exactly what it is.
What Is Electrolysis? The term "electrolysis" has come to be very loosely applied
in the small-boat field. What many boat-men call "electrolysis" is often really
some kind of corrosion.
Depending upon your dictionary, you will get all kinds of definitions from the
simple "The decomposition of a chemical by an electric current," to a fat paragraph
in Webster's New International Dictionary dealing with electrolytes, ions, and Faraday's
laws.
In the strict sense of the term, electrolysis concerns chemical changes in the
solution (in this case, the salt water) due to the passage of current. Since we
need a term to work with, however, let's call the corrosion of metal parts involved
in electrolysis "electrolysis corrosion."
When Does It Occur? Electrolysis corrosion occurs when direct current, as from
a battery or generator, passes through the water from one conductor to another.
You can get a good example of this activity very quickly and easily.
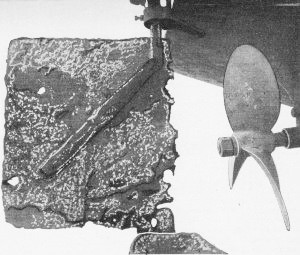
Severe galvanic corrosion in a steel rudder connected to copper-bottom
sheathing, bronze shaft and propeller. The boat almost became a casualty.
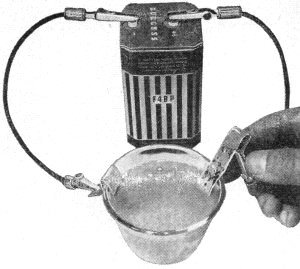
Electrolysis corrosion can be studied by immersing copper strips
in brine, and connecting a battery. In a short time, the current flow will cause
the positive electrode to corrode - eventually be entirely eaten.

"Self-devouring" propeller at right suffered from faulty alloy,
so zinc block, mounted on wood, above, gave no protection.
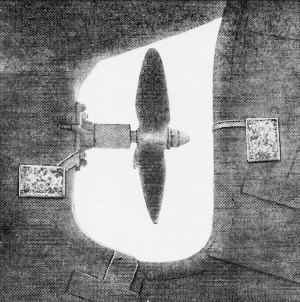
Zinc blocks must be connected to metal that they are to protect.
This propeller nut has a zinc overcoat. Blocks on deadwood and rudder are connected
by copper straps to rudder post and stern bearing.
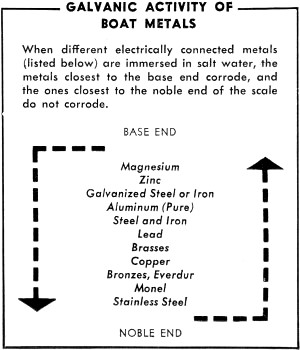
Galvanic Activity of Boat Metals
When different electrically connected metals (listed below) are
immersed in salt water, the metals closest to the base end corrode, and the ones
closest to the noble end of the scale do not corrode.
Dunk a pair of copper strips in a cup of salt water (table salt will do), and
connect them to a 6-volt battery. Bubbles boil from the negative electrode and nothing
much happens at the positive one - except that you may observe a green cloud creeping
out from it into the water. What is happening is that the metal of the positive
strip is going into the solution. In a short while, the weight loss of this electrode
can be measured with a sensitive scale. Carryon your experiment long enough and
there won't be any positive-end copper left.
This is what boatmen commonly call electrolysis - loss of metal due to battery
current between underwater parts. Run battery current between a boat's under-water
metal parts, and the positive part corrodes rapidly away. Stop the current flow,
and the process halts.
Since no one would reasonably connect a battery between underwater metals on
his boat, how can this possibly happen? Very easily, due to the fact that one side
of the battery circuit for motors and many electrical boat accessories and lights,
etc., is connected to the frame, shell, or "ground."
If you cross-ground one gadget by using opposite ground polarity from that used
on any other fixture, you've set up your boat for possible electrolysis corrosion.
The damaging current flow can take place through bilge water as well as through
the water under the boat; and it has also been known to take place through wet wood.
So, when installing fixtures, taking batteries ashore for charging, or doing anything
with the electrical system of the boat - keep the polarity of the ground connections
the same. Lately, manufacturers have begun to standardize to a large degree on negative
grounding; but don't count on it - investigate and be sure.
Another way to invite electrolysis corrosion is to use insufficient heavy wire
to supply power to heavy-drain items, such as a radiotelephone, which have ground
connections to the water. Voltage drop occurring in the ground leg of the power
supply circuit is just the same as with battery voltage: if there is a difference
of potential between points A and B under-water, no matter if the voltage is from
a generator, battery, or a voltage drop in a wire, trouble can result. Use large
wire for power leads to keep voltage drop close to zero. In addition, it is a good
idea to connect grounded objects together inside the boat with a "bonding" wire
of at least No. 10 gauge, and also bond in the engine frame and radio ground plate,
if any.
Galvanic Corrosion. Most underwater corrosion has no connection whatever with
electrolysis, contrary to popular thought. This trouble is simply "galvanic corrosion."
It takes place when three conditions are satisfied: the metals are in contact with
salt water; they are different in composition; and they are in electrical contact
through a metallic path: This forms a galvanic cell - like a primary battery cell.
Current is generated, flows through the water, and the metal supplying the current
corrodes.
This automatically happens when ordinary hardware-store brass is used for underwater
fittings or fastenings on a boat. Ordinary brass is an alloy composed of about 30%
zinc and 70% copper. The table below the galvanic activity of boat metals. Note
that zinc is at one end of the scale and copper near the other. Result: the zinc
is corroded, leaving a spongy copper mass in place of the brass. This has no strength,
so holes appear and fastenings disappear.
Many alloys are so cannibalistic that they will feed on themselves in this fashion.
So, the first rule which must be followed to avoid galvanic corrosion is to use
only fittings and fastenings which are made of seaworthy metal: bronze, Everdur,
Monel, stainless steel, and - if you are loaded - titanium, gold and platinum. All
are extremely resistant to corrosion.
Next, make sure that all of the different underwater metals are of the same family.
Avoid galvanized iron or steel if there is copper or any of the other more noble
metals around. Always try to use fastenings. which are more noble than the metal
of the object they are holding in place. This way, if there is the least bit of
corrosion, it will affect the comparatively bulky piece of hardware (which can afford
to lose a few grams weight) instead of the smaller fastening which may weaken to
the point of failure after the loss of just a little metal.
An outboard motor is, unfortunately, mostly active metal, and likely to corrode
quite rapidly if given the right conditions. To be on the safe side, when your motor
is not actually in use, keep it up out of the water ... especially if it has a bronze
propeller.
The importance of avoiding unlike combinations of metal underwater cannot be
stressed too much. And never install an object made of a metal about which you are
unsure.
The builders of a beautiful yacht, the "Sea Call," learned this the hard way.
Monel is practically proof against corrosion, so they built the underwater shell
of pure Monel. But the frames, stem and rudder were steel; and while most of the
rivets were Monel, a few steel rivets were accidentally mixed in. Shortly after
the vessel was placed in the water, one of these steel rivets disappeared, and people
began to get suspicious. Upon dry-docking the yacht, it was found that all of the
steel near the Monel metal was rapidly corroding, while the Monel, of course, remained
unaffected. The yacht was built in 1915 and scrapped in 1916!
Cures for Corrosion. Prevention is the best cure for corrosion, of course, but
many times it is not possible to have conditions as perfect as would be desired.
Good underwater metals may pick up dirt or oxide which changes their galvanic activity;
small impurities in a metal can create galvanic hot spots; and it is conceivable
that somewhat different metals must, of necessity, sometimes be used. On large ships,
you will often find combinations of aluminum and steel or bronze.
If it is necessary to use different metals, they must be electrically insulated
from each other. Plastic gaskets, Micarta separators and sleeves, rubber or other
water-proof insulating material can be used. As long as there is "no circuit," corrosion
will stay away.
You can also obtain protection with unbroken coverings of plastic paint, neoprene
compound, or other insulating coverings.
But the most popular method of protecting metals which unavoidably suffer from
galvanic corrosion was cooked up by Sir Humphrey Davy in 1824. The copper sheathing
on warships was corroding. "Attach blocks of zinc to the copper," said Sir Humphrey.
The idea was that, being more active than any surrounding metal, the zinc would
supply metal for all of the galvanic action, and the other metal would remain unscathed.
This idea was so good that even today U. S. Navy vessels which must be as light
and maneuverable as possible have tons of zinc anodes attached to their hull plating.
Special zinc anodes are made for small boats in the form of plates, propeller-nut
caps, and shaft sleeves, and are available from marine hardware stores. The zinc
must be as pure as possible (less than .0014% iron content), and it must be attached
or electrically connected to the metal which it is supposed to protect. It must
not be painted, however, and when it has corroded badly (it will corrode, if it
is providing any protection), the zinc anodes must be replaced.
A more modern method of protection is to supply a reverse current from a platinum
anode fitted to the hull, powered by a battery-operated regulated supply. Although
this is highly effective, it is somewhat more expensive.
The Scapegoat. Underwater metal comes apart for many more reasons than electrolysis
and corrosion. Among these are abrasion from mud and sand, and cavitation.
At the time when comparatively little was known about the installation of electrical
and electronic equipment on small boats it was fashionable, and very easy, to blame
every bit of underwater trouble on electrolysis. Radio had a bad name. Sober-faced
"experts" actually claimed that the instant you installed a radiotelephone on a
boat, the underwater hardware would start to fall off.
But since ghosts have gone out of vogue, don't tremble at the awful specter of
electrolysis. Keep track of your electrical circuits so that current does not flow
through the water; use sea-going metals and be sure they are properly mated; and
use zinc anodes, if necessary. Take these basic protective measures, and you should
have no trouble.
Posted July 16, 2019
(updated from original post on 10/26/2011)
|

























 How to save your boat from the ravages of electrical
corrosion
How to save your boat from the ravages of electrical
corrosion 




