July 1959 Popular Electronics
 Table
of Contents Table
of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
"Mr. Scott, prepare to load
the promethium batteries into the
dilithium crystal chamber." OK, I made up the promethium batteries
part, but you might not have suspected it. Back in the mid to late 1950s, atomic
batteries were seriously thought to potentially (no pun intended) be a futuristic
source of energy storage and generation. The concept worked by having beta particles
from promethium decay impinge on silicon photodetectors, and having that be the
source of power. That's almost as Rube Goldberg-ish as having a gasoline engine
drive an electric generator to power a motor for automotive locomotion. Oh, wait,
that describes the Chevy Volt.
Note that in 1959, nickel cadmium (NiCad) batteries were just coming into commercial
use, and the author envisions a day when they might be used for portable power tools
and flashlights. At least he was right about that one.
See the 1957 edition of Time magazine with an article titled, "Science: New Atomic Battery."
Recent Developments in Battery Design

Two soldiers at a front-line observation post crouch behind a
strange-looking device that looks like a spotlight: As they wait in the darkness,
instead of straining their eyes to discern enemy movements, their attention is riveted
to the spotlight-like device. Capable of spotting a single enemy soldier a half-mile
away in total darkness, this is the Army's new "Silent Sentry" mobile radar set.
Atomic batteries, fuel cells, and other new battery types hold great
promise for the future
by Saunder Harris, WINXL
Under battle conditions quiet operation of the Silent Sentry could be a matter
of life or death; therefore, a noiseless source of power for the unit is essential.
Obviously, here is a job tailor-made for batteries. But which type should be used?
Of a whole parade of new batteries, the Army has chosen one of the newest-the little-known
fuel cell-to power the Silent Sentry.
The fuel cell is only one of the new battery types which are today proving their
worth. Already on the commercial market are the tiny, power-packed mercury cell
and the rechargeable nickel-cadmium battery. Before long, the fuel cell, too, will
be available to private citizens. And the most fascinating development in batteries
- the amazing promethium cell which is powered by energy from the atom itself -
is now be-ing tested and refined in research laboratories in this country.
Let's examine these "wonder" batteries. First, since it's one of the newest,
we'll take a look at the fuel cell, the power source of the Silent Sentry.
Fuel Cell

The scientist who did much of the research on the fuel cell,
Dr. Karl Kordesch, examines an electrode used in the cell.
The most interesting characteristic of the fuel cell is that, unlike conventional
batteries, it never becomes exhausted. Since it produces electrical current from
the electrochemical reaction which takes place when oxygen and hydrogen are combined,
the fuel cell itself remains usable as long as the "fuel" - oxygen and hydrogen
- is supplied.
Operation of the fuel cell is diagrammed in Fig. 1. Oxygen and hydrogen
enter the cell through two hollow carbon electrodes. Since these electrodes are
porous, the gases rapidly diffuse to the outer surface of the electrodes where they
come into contact with the electrolyte, a solution of potassium hydroxide. The chemical
reaction which takes place releases electrons from the hydrogen electrode which
flow through the external circuit and are returned at the oxygen electrode. It is
this flow of electrons which provides the electric current. Water, a by-product
of the reaction, is passed from the cell in the hydrogen stream.
One fuel cell can produce only about one volt. Any required voltage may be attained,
however, by simply connecting the cells together in series. As with ordinary dry
cells, the amount of current which can be drawn from a fuel cell is a function of
its physical size. Thus, by varying the number and size of the cells, many variations
of voltage and current can be obtained. Fuel cells have been operating eight hours
a day, five days a week, for over a year with no sign of deterioration.
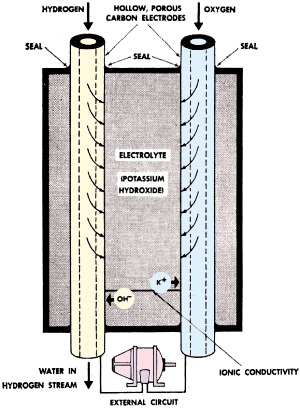
Fig. 1 - Operation of the fuel cell. Electron flow from
hydrogen electrode to oxygen electrode provides electric current.

Nickel-cadmium and mercury batteries such as these are already
on the market.
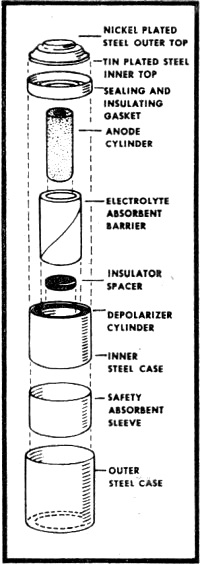
Fig. 2 - Exploded view of mercury battery (courtesy National
Carbon Company)

Fig. 3 - Comparison of discharge curves of mercury cell and carbon-zinc
cell. It is readily seen that the mercury cell enjoys a much longer useful service
life.
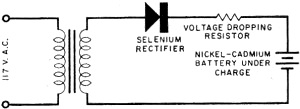
Fig. 4. Typical simple rectifier circuit employed to reactivate rechargeable
batteries.

Fig. 5 - Promethium cell composed of center layer of promethium and phosphor
with outer layers made up of photocells.
It is very possible that the fuel cell will be the practical means of putting
both nuclear energy and solar energy to use. At present, one of the big difficulties
involved in using the energy of the sun is in storing its power for future use.
Now, during the sunlight hours, the sun's energy could be used to decompose water,
producing both hydrogen and oxygen for later use in fuel cells. In the same manner,
where nuclear reactors are used as heat sources in steam generating plants, the
nuclear energy decomposes water. Instead of this being a disadvantage, as it has
been, this process can now be the means of producing the necessary hydrogen and
oxygen for fuel cell operation.
Mercury Batteries
Wherever long life and lots of punch must be jammed into a small package, the
mercury battery has come into wide use. This battery was developed primarily for
hearing aids and other ultra-miniature equipment.
An exploded view of a typical mercury battery is shown in Fig. 2. The materials
used in its construction are high-purity zinc powder for the anode, mercuric oxide
and carbon for the cathode, and potassium hydroxide as the electrolyte. The mercury
cell develops an open-circuit voltage of approximately 1.35 volts.
Figure 3 illustrates the difference in performance between the mercury cell and
the standard carbon-zinc cell. It can be readily seen that the voltage of the mercury
cell remains constant over a longer period of time than does the carbon-zinc cell.
For general use, the mercury battery is very expensive. The size "D" mercury
battery (flashlight size) costs about $2.50 as compared with a price of 20 cents
for an ordinary carbon-zinc "D" cell. This high cost is due to the expensive materials
used in construction. Mercury batteries became financially practical only when devices
such as hearing aids were designed to use them.
Nickel-Cadmium Battery
Along with other dry cells, the mercury battery suffers from one big disadvantage.
It cannot be recharged. However, rechargeable nickel-cadmium batteries are just
coming into popular use today in rechargeable flashlights, radios and electric razors.
Tomorrow they may be used to power TV sets, portable electric drills, and perhaps
even your car.
After conventional dry cells have been used awhile, the action of the cell is
gradually choked off by a gas which is developed in the cell. In the nickel-cadmium
battery, the recharging process converts this gas back into a liquid, thus reactivating
the cell. Recharging is accomplished by plugging the battery unit into the house
power line. Figure 4 shows a typical half-wave rectifying circuit used for this
purpose. In most cases, the rectifier is built into the same unit as the battery.
Nickel-cadmium cells can't give you something for nothing, however. Rechargeable
cells cost more, and give less energy per charge than an ordinary carbon-zinc cell.
For example, a nickel-cadmium flash-light cell costs about $2.75 as against 20 cents
for a comparable carbon-zinc cell. But it can be charged over and over.
Even more expensive than the nickel-cadmium battery is its highly refined "cousin,"
the silver-cadmium battery. This battery enjoys all the advantages of the nickel-cadmium
design, and, in addition. offers higher output at one-half to one-third its size
and weight. Since the silver-cadmium battery is quite costly, its greatest application
so far has been in rockets. missiles, and satellites.
Atomic Batteries
Much misleading publicity has surrounded the atomic battery. In spite of newspaper
reports which would lead you to believe that an atomic powered radio is just around
the corner this is not the case. As one battery engineer put it, "there are a great
many problems to be solved before atomic batteries are brought out of the laboratories
and put into your home."
One objection to the atomic battery is its potential danger. How would you like
to have a little package of radioactivity around the house where it might be broken
into by youngsters with a yen for experiments? Another objection is cost; at this
time, the material which goes into such batteries is extremely expensive. The batteries
we have working for us now do a good job at reasonable cost and the idea of replacing
them with atomic batteries might be novel but cannot be considered practical at
present.
However, laboratory research continues on the atomic battery. Radioactive promethium
- promethium is a by-product of uranium fission - is the power source. It is valuable
because it emits large amounts of beta rays (actually electrons) over its 2 1/2-year
half-life. These beta rays can be tapped as a source of power. Alpha a gamma rays
are emitted only in small quantities.
The actual size of the promethium and its shielding is about that of a penny
Figure 5 shows a typical promethium cell in cross section. The center layer is a
mixture of promethium and phosphor. Small photocells compose the outer layers. When
the promethium gives off beta rays, they strike the phosphor with great force. The
phosphor then lights up in much the manner as your TV screen does when the electron
stream of the cathode-ray tube hits it. This light is then converted to electrical
energy by the two outer layers of photocells.
Output of the promethium cell is small - actually less than one-millionth of
the electrical power used by a 40-watt bulb. It does give off power, though, and
the power comes from atomic radiation. Considering its early stage of development,
the promethium cell shows great promise.
Any discussion of batteries must necessarily lead us back to the fact that the
carbon-zinc cell is still battery king of the present. And in the distant future,
when you send one of the kids down to the Lunar Hardware on the Moon to pick up
a battery for your flashlight, chances are you will end up with our old friend,
the carbon-zinc cell.
Posted October 6, 2022
(updated from original post
on 10/11/2011)
|
















