October 1960 Popular Electronics
 Table
of Contents Table
of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
 Looking at the first
picture reminds me of a scene in Star Trek VI
where Mr. Chekov takes a fall while exploring a "nuclear wessel" and is left
unconscious. By the time Capt. Kirk and Dr. McCoy get to him, the doctors
have prepared Chekov for 20th-century-style brain surgery. McCoy is abhorred over
the "midievalism" technique that actually requires cutting open the skull to treat
the injury. Instead, the good doctor passes his humming little device over Mr. Chekov
and heals him instantly. Someday, if mankind or an asteroid doesn't wipe out civilization,
we probably will reach the point of Dr. McCoy's magic brain healing gizmo. I
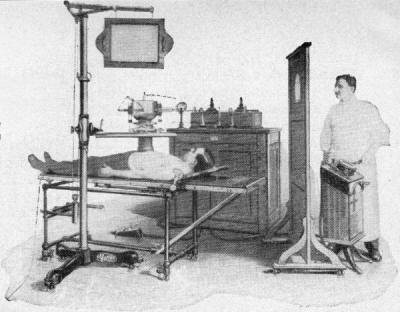
will reassert the "barbaric" description here regarding how scary-looking early medical x-ray
machines were as shown in the October 1960 issue of Popular Electronics
magazine. Admittedly, being stuffed inside a modern MRI machine and having the loud
electromagnetic coils cycling is no relaxing trip to the countryside, but the
early machinery was downright frightening. See also "Electronics Against
Cancer" for more info. Looking at the first
picture reminds me of a scene in Star Trek VI
where Mr. Chekov takes a fall while exploring a "nuclear wessel" and is left
unconscious. By the time Capt. Kirk and Dr. McCoy get to him, the doctors
have prepared Chekov for 20th-century-style brain surgery. McCoy is abhorred over
the "midievalism" technique that actually requires cutting open the skull to treat
the injury. Instead, the good doctor passes his humming little device over Mr. Chekov
and heals him instantly. Someday, if mankind or an asteroid doesn't wipe out civilization,
we probably will reach the point of Dr. McCoy's magic brain healing gizmo. I
will reassert the "barbaric" description here regarding how scary-looking early medical x-ray
machines were as shown in the October 1960 issue of Popular Electronics
magazine. Admittedly, being stuffed inside a modern MRI machine and having the loud
electromagnetic coils cycling is no relaxing trip to the countryside, but the
early machinery was downright frightening. See also "Electronics Against
Cancer" for more info.
After Class: X-Rays
 By Fred E. Ebel, W9PXA By Fred E. Ebel, W9PXA
Discovered by chance only 65 years ago, X rays are one of our most valuable research
tools
Is it light?
No.
Is it electricity?
Not in any known form.
What is it?
I don't know.
With such scientific frankness did physics professor Wilhelm Konrad Roentgen
relate his discovery of a mysterious new ray to a newspaper reporter. Stumbled upon
by accident during a routine laboratory experiment on the night of November 8, 1895,
the new ray was dubbed an "X ray" by Roentgen. "X," then as now, was the mathematical
symbol for the unknown.
Much of the "X" has been taken out of X rays since the modest German physics
professor demonstrated this startling radiation and its power to penetrate tin,
paper, wood, and even the human body. When X-rays were first put into use, you ordinarily
had to visit a hospital to see them in action. Today, unlimited industrial applications
make X rays far more than just a diagnostic and therapeutic tool of medical science.
 Chance - or Fate? Just
what did happen on the night of November 8, 1895? Call it fate, fortune, or chance,
but there were a number of conditions that conspired to make this night one to remember.
First, Roentgen had completely covered the Crookes tube he was using with a black
cardboard, making it light-tight. Secondly, his laboratory itself was plunged in
darkness. Finally, the piece de resistance - a sheet of paper painted with crystals
of barium platinocyanide - lay on a bench some distance from the tube. Chance - or Fate? Just
what did happen on the night of November 8, 1895? Call it fate, fortune, or chance,
but there were a number of conditions that conspired to make this night one to remember.
First, Roentgen had completely covered the Crookes tube he was using with a black
cardboard, making it light-tight. Secondly, his laboratory itself was plunged in
darkness. Finally, the piece de resistance - a sheet of paper painted with crystals
of barium platinocyanide - lay on a bench some distance from the tube.
The barium platinocyanide screen was the "chance" that nature gave Roentgen to
unlock one of her secrets. For when the crystals glowed with a shimmering yellow-green
fluorescence, Roentgen's keen scientific mind became curious. True, cathode rays
could make the crystals glow - but at this distance? He placed the crystal screen
at an even greater distance from the tube than the range cathode rays were known
to penetrate. Still the strange fluorescence!
Heart pounding, he grabbed a book and placed it between the Crookes tube and
the screen. The crystals continued to glow. Whatever it was, it was coming through
the book! Next, he tried metals - and found that the rays penetrated in varying
degrees, although lead and platinum stopped them completely.
Now came the most dramatic test of all.
 Roentgen exposed his hand, and - his heart
must have almost stopped - saw the shadows of his bones. As Roentgen made photographs
of his findings, it was obvious that he had found something far more exciting than
cathode rays - X rays! Roentgen exposed his hand, and - his heart
must have almost stopped - saw the shadows of his bones. As Roentgen made photographs
of his findings, it was obvious that he had found something far more exciting than
cathode rays - X rays!
How X Rays Are Formed. How was Roentgen able to produce these
powerful rays with his crude apparatus - a modified Crookes tube, mercury interrupter,
and a Ruhrnkorff induction coil that furnished a bare 20,000 volts? The simple fact
is this: X rays are relatively easy to generate. You simply speed up electrons and
let them collide with a target. The electrons cause disturbances within the atoms
of the target, releasing X rays. Any material, even a gas or liquid, will release
X rays when bombarded by high-velocity electrons.
Obviously, then, glass can be a target, as is was in the Crookes tube Roentgen
used - see Fig. 1. Here a gas-type tube, consisting of an anode and cold cathode,
was connected to a high-tension induction coil. Heavy positive ions in the residual
gas were drawn to the negative cathode (unlike charges attract), striking with such
force that they knocked electrons from the cathode metal. It was this positive-ion
bombardment that created and maintained a source of electrons, the "work horses"
for X-ray generation.
The negatively charged electrons, in turn, were drawn toward the high-voltage
positive anode. The resultant stream of electrons, actually cathode rays, traveled
so fast - about 30,000 miles per second - that most of them could not "turn the
corner" to reach the anode. Instead, they smashed into the glass wall of the tube.
The glass, therefore, was the target, providing the barrier for the sudden stoppage
of electrons. The result: radiation of X rays, and, of course, the glow of fluorescence
of the glass that Roentgen observed.
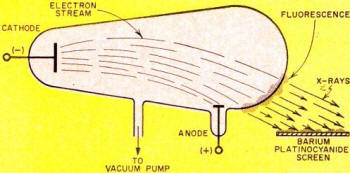
Fig. 1 - Crookes tube used by Roentgen produced X rays when
electrons flowing from its cathode to its anode bombarded the glass tube.

Fig. 2 - Basic X-ray unit includes X-ray tube and high-voltage
supply.
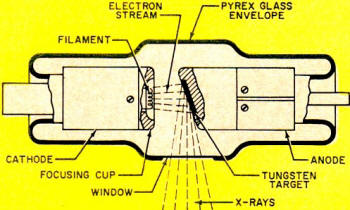
Fig. 3 - X-ray tube detail. The focusing cup concentrates
electron stream from cathode and directs it toward tungsten target material.
Nature of X Rays. X rays are electromagnetic rays similar to
visible light rays, with this important exception - their wavelength is very small,
about 1/10,000th that of light. These tiny wavelengths are measured in angstroms,
units so small that you can line up 254,000,000 of them between the one-inch marks
on a ruler. The X-ray region in the electromagnetic spectrum ranges from about 0.006
to 1000 angstrom units. Interestingly enough, it is this exceedingly short wavelength
of X rays that makes possible their penetration of matter, and which enables the
researcher to delve into the vast voids of molecular inner space.
Unlike the cathode ray generated in your TV picture tube, X rays are non-electrical.
Thus, they are unaffected by electrostatic and magnetic fields. This can be proved
by placing a magnet or charged plate near X rays; they "ill be neither attracted
nor repelled as in the case of cathode rays.
Traveling at e same speed as light and radio waves - 186,000 miles per second,
X rays can be reflected and refracted only at very small angles. (Roentgen failed
to focus X rays, despite many experiments with lenses of wood, glass, aluminum,
and other materials, for this reason.)
The darkening of photographic film by X rays has given them wide application
in medicine, research, and industry. A radiograph used by makers of cast-metal products
is actually a shadow picture of the subject. The dark regions of the film represent
the more penetrable parts - gas pockets in a weld, for example; the lighter regions
identify the more opaque areas.
How X Rays Work. A basic X-ray unit is comprised of filament,
high-voltage transformer and timing circuits - see Fig. 2. The heart of the
unit is the X-ray tube. Like Roentgen's original tube, the modern tube also has
a cathode and an anode, but with tremendous improvements. Now the tube is evacuated
to an extremely high vacuum. The cathode structure contains a coil of tungsten wire
- the filament - which "boils off" electrons when heated to incandescence. A metal
reflector or focusing cup on the cathode directs the electron beam toward the target-as
shown in Fig. 3.
Tungsten is ordinarily used for the target material, since it can withstand high
temperatures without melting. This is important because less than 1% of the energy
in the electrons is converted to X rays upon bombardment with the target; most of
the energy is converted to heat. To help dissipate the heat, the tungsten is imbedded
in a large mass of copper which conducts the heat into air or into oil, as in the
case of the oil-immersed tube.
It is desirable to have the focal spot - the area of the target that receives
the electron bombardment - as small as possible. The smaller the focal spot, the
better the detail of the radiograph. But a small focal spot means an intense blast
of electrons in a tiny area; even tungsten melts under such grueling treatment.
This problem can be solved by simply rotating the anode target. The target constantly
turns another "face" to the electron stream, area - see Fig. 4.
An induction motor provides the rotating power in an ingenious way. The stator
surrounds the outside of the evacuated glass bulb tube and provides the rotating
magnetic field that turns the rotor in the tube at approximately 3000 rpm. The rotor
in the "neck" of the tube is, of course, connected to the target. The entire moving
assembly is located inside the evacuated tube.
The high-voltage circuit consists of a step-up transformer and its controls;
an autotransformer supplies voltage to the primary winding of the high-voltage transformer.
Any change of the autotransformer voltage produces a corresponding change in the
high-voltage output which is applied to the X-ray tube. Changes in voltage are made
with a selector switch; increasing the tube voltage results in a decrease in wavelengths
of X rays, accompanied by an increase in penetrability.

Fig. 4 - Rotating anode in target constantly turns new face
to electron stream in order to distribute heat over a wide area.
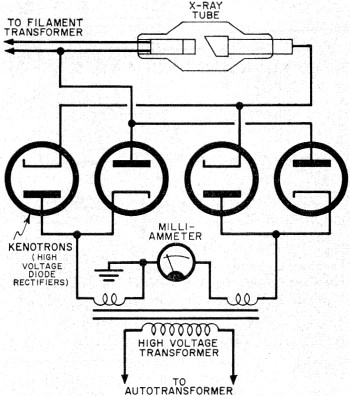
Fig. 5 - High-voltage rectifier circuit used in some X-ray
systems.
If "soft" X rays of low penetrability and longer wavelengths are desired, the
selector switch is set at about 20,000 volts. But if "hard" X rays of high penetrability
and shorter wavelengths are desired, the switch is set for several hundred thousand
volts.
Rectification of the high-tension alternating current to the tube can be very
simple - in fact, the circuit can be made self-rectifying. Current will flow through
the tube only on the half cycle when the anode is on its negative half cycle, since
the anode now repels the negative electrons. Some X-ray systems use a high-voltage
full-wave rectifier circuit, permitting conduction of current through the tube on
each half cycle of alternating current-see Fig. 5.
Present Day Uses. Quality control in manufacturing makes extensive
use of the non-destructive quality of X rays in the inspection of casting and weldments
for such defects as cracks and gas pockets. X-ray devices in beverage plants "look
into" opaque cans moving rapidly on a conveyor line and give the signal for automatic
rejection of under-filled cans.
Similar devices reveal foreign bodies in food stuffs; detect the hollow heart
of potatoes; separate pithy from juicy oranges; and reveal the improper assembly
of electronic tubes, switches, and small electrical assemblies. X rays also gauge
the thickness of electroplating, as well as that of hot steel strip racing along
at 4000 feet per minute in a rolling mill.
Dramatic applications abound in X-ray diffraction. Here, X rays are made to bounce
off mirror-like atomic planes of crystalline substances to reveal secrets of inner
structure. Nylon, magnetic TV tape. synthetic rubber, high-temperature alloys, high-test
gasolines, and penicillin are just a few of the products X-ray diffraction has helped
to develop or improve.
Art museums use X rays to examine the authenticity of old paintings. In other
applications, X rays distinguish real diamonds and pearls from their imitations.
Biologically Speaking. It is now well known that X rays as well
as gamma rays can mutate or change the genes (hereditary units) of our bodies. Excessive
X-radiation can also affect flesh, bone, and blood destructively. For these reasons,
it is of utmost importance that exposure to radiation be kept at a minimum.
What can be done in this respect? So far as background radiation is concerned,
even Adam and Eve had to contend with the small amount of gamma radiation from radioactive
material which occurs naturally in soil, rocks, and even plants. In fact, there
are radioelements in our bodies that give each of us a daily unavoidable radiation
dose of 0.0001 roentgen. (The roentgen is the unit of X- and gamma-ray dose.) In
addition, cosmic rays from interstellar space add to our daily dose of background
radiation.
In essence, X rays are simply a form of man-made radiation, but new techniques
and advancements greatly reduce the effects of their exposure to patients. Diagnostic
voltages now up to 150 kv. permit much shorter exposure times, as do faster films.
Significant, too, are collimators that confine the X-ray beam to the exact area
desired.
All in all, few would deny that the tremendous diagnostic and therapeutic benefits
of X rays far outweigh any possible deleterious effects. In fact, many a man, woman,
and child is alive today because of Roentgen's startling discovery. Since that eventful
night in 1895, these once strange and unknown rays have done much to alter the nature
of the world we live in.
Posted June 18, 2021
(updated from original post on 5/20/2014)
|


























 By Fred E. Ebel, W9PXA
By Fred E. Ebel, W9PXA 






