|
April 1944 Radio-Craft
 [Table
of Contents] [Table
of Contents]
Wax nostalgic about and learn from the history of early electronics.
See articles from Radio-Craft,
published 1929 - 1953. All copyrights are hereby acknowledged.
|
The probability-based quantum mechanical
model of atoms has been in existence since around 1932 when
Robert Mulliken
coined the term "electron
orbital." It superseded the
Bohr model that modeled the atom as a proton/neutron
nucleus that was surrounded by electrons orbiting like planets around a star. For
many decades thereafter, text books - particularly those used in beginner level
courses - continued to present the Bohr model and only gave passing reference, if
at all, to the quantum model. The Bohr model was and still is easier for most people
to envision, although as time goes on the percentage of people who even recognize
a planetary model is probably rapidly decreasing. This article from a 1944 edition
of Radio-Craft magazine chooses to use the Bohr model as part of an introduction
to electronics. Today, you might need to start from a lower point and talk about
groupies swarming around rock stars for most people to not give you the deer-the-the-headlights
look.
A Short Course in Practical Electronics
Lesson I - The Electron
By Fred Shunaman
Beginning with the fundamental electron, this course. discusses, electric phenomena
as a result of electron activity. This may be a welcome innovation to students who
have had difficulties with traditional presentations.
The electron - that infinitesimal; invisible particle of pure electricity - the
basis of all matter - is logically enough the starting point of any study of electronics,
Our grasp of all electric circuits will be remarkably simplified by looking at them
as the result of such activity of electrons. Electricity and Radio studied from
·the electronic viewpoint become reasonable and easy to understand.
What is the electron? We are informed that it, is the smallest particle of matter
(so far accepted). According to another definition, it is the "unit charge of negative
electricity." Neither of these definitions describes the electron, though they might
lead us to suspect correctly that there must be a close connection between matter
and electricity. It is as if we were told that Joseph Doakes is a Presbyterian,
and in another definition that he weighs 150 pounds. Both descriptions might be
correct, but would not help us to recognize Joe. about the electron, we must dig
into the structure of matter itself.
Scientists have convinced themselves - by methods extremely interesting but impossible
to describe in this space - that all matter is composed exclusively of positively
charged protons an negative electrons. (It would be a mistake to say "negatively
charged" electrons. An electron is a negative charge - a small piece of negative
electricity.) This statement is over-simplified, leaving out such bodies as the
recently discovered positrons and neutrons, but is sufficiently accurate for the
purposes of our study.
Electron and Proton

Fig. 1 - How the macroscopic copper cent would look to a
wanderer from the outside world.
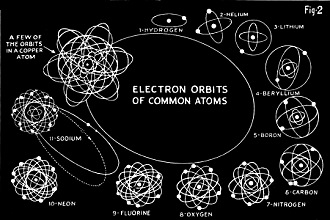
Fig. 2 - Electron shells of atoms from hydrogen to sodium,
also copper. The long orbits are the "wild" electrons of copper and sodium.
According to this view, each of the atoms of which all matter is composed consists
of a central nucleus made up of one or a number of positive protons (with a few
electrons bound tightly into the mass) around which revolve one or a number of electrons,
much in the manner of the Earth around the Sun.
Indeed, the Earth revolving around the Sun is a fair picture. It describes something
of the spaciousness of solid substance. For the most astounding thing about matter
is its very lack of matter. The most solid substance known is far more,. than 99.9%
nothingness. If we were to take an ordinary copper cent - to use an ancient illustration
- and expand it to 100 million miles in diameter, increasing its thickness at the
same rate, we would be able to see those atoms and electrons of which we speak.
The nucleus of the atom of copper, thus magnified, might be a little larger than
a baseball. Each of these atom centers in our enlarged cent, would be more than
a mile from its nearest neighbor, the center or a sphere a mile in diameter. This
whole area would contain only 29 electrons revolving in various orbits around the
nucleus. An explorer in this copper universe would view a scene like that of Fig. 1.
These 29 electrons are held in their places by the attraction of the positive
nucleus, and kept from each other by the mutual repulsion of one negative charge
for another, We are all familiar with the phenomena of static electricity - how
a glass rod .which has had a few electrons rubbed off it by friction against a piece
of paper will try to drag bits of lint, silk or tissue paper toward it. It is left
with a positive charge, and wants its electrons back again. To get a few electrons,
it, will drag the relatively enormous piece of paper distances which much appear
astronomical to the electron.
The atoms are in their turn kept apart by the repulsion between the flying. electrons.
This force of repulsion is so great as to be hard to describe in terms of the ordinary
forces to which we are accustomed in daily life. It has been calculated that
if 2½ pounds of electrons were located at the North Pole and an equal, quantity
at the South Pole of the Earth, they would repel each other with a force of 200,000,000,000,000
tons! With forces like that inside it, it is not surprising that the copper penny
does not collapse into itself.
Seriously, this openness and emptiness of matter is very important. It gives
the electron a chance to get around. When bound to its nucleus by electric attraction,
it is a part of electrically neutral matter. But if some way is devised to get it
moving on its own, it becomes electron flow or current, and temporarily seems to
be something altogether different from matter.
How the electron may be persuaded to start out independently is also bound up
with the way atoms are constructed. Though all atoms are composed of protons and
electrons, there are no less than 92 primary varieties of them, the 92 elements
from which all substances on earth are made. The electrons in all these 92 varieties
are exactly alike, but the number varies from 1 - in hydrogen, the smallest and
lightest atom of all-to 92 in the atom of uranium. (Besides its 92 planetary electrons,
revolving outside the nucleus, uranium has an additional 146 packed inside, the
whole balancing the 238 protons it contains.)
These planetary electrons revolve around nucleus in well-defined orbits, called
shells. No more than two electrons are found in the inner shell. Helium is the atom
with 2 electrons, and. is the second lightest thing in the world. Next up in the
scale is lithium, the lightest solid substance. It also has its two electrons, and
in another orbit far outside that of the first two, a third one. As we go on through
the list of elements, each one adds another atom to the outer shell till we come
to the familiar neon, which has an outer shell of 8 electrons. At the next atom,
sodium, we get another break. The 8-atom second shell remains intact, and another
lonely electron is away out in space, trying to start a third one. Apparently not
more than 8 electrons can crowd into the second shell.
As elements "become .more complex in structure, successive shells are built up,
till we find the electrons of the uranium atom revolving in seven shells, with 2,
8, 18, 32, 18, 13 and 1 atom in each, as counted from the inner one.
The outer shell of an atom decides its chemical or electrical disposition. Peculiar
among all the elements, are those contented atoms with full outer shells. Helium
has long been known to be the ideal gas for dirigibles, because it will combine
with no other element. Unlike hydrogen, which is always searching for another substance
to fill the empty place in its outer shell, and combines furiously with the oxygen
in the air on the least provocation, helium cannot be burned or otherwise affected.
chemically.
Atoms with incomplete outer shells tend to be more sociable, combining with others
which will help them fill the vacant outer spots in their envelopes. Fluorine, only
one electron short of the eight needed for a complete outer shell, is such an inveterate
joiner that it long escaped discovery. It combined immediately with the glass in
the chemists' vessels, and was never to be found in the substances being investigated!
Even more interesting are those atoms with only one or a few electron in outer
shell. The lonely electrons, whose bond to the nucleus is comparatively weak, may
be knocked loose from its parent atom by collision with another electron, or by
other means. We then have a free electron. The atom, now short one electron, becomes
a positive ion. If some means can be found to knock the outside electrons off a
great many atoms and impel them in a certain direction through an element, we have
an electron flow or electric current.
Elements (or compound substances) through which electrons can flow easily are
conductors of electricity. Silver, the best of all conductors, and copper, the structure
of which is shown on the opposite page, most commonly used to carry electricity,
both belong to the group of elements which have one electron in the outer shell,
as does gold, another excellent conductor.
Metals such as zinc and aluminum, which have more than one electron in the outer
shell, may also be good conductors.
Substances which have complete outer shells might be expected to be good insulators.
Quartz is one of the best insulators known. It is a combination of two atoms of
oxygen with one of silicon. Silicon has four electrons in an outer shell with room
for eight-oxygen lacks two to complete its outer shell. The four outside electrons
of the silicon atom fill in the spaces in the two atoms of oxygen in such a way
as to produce a singularly contented molecule - one into or out of which it is next
to impossible to drive an electron. Many substances, such as glass, certain plastics,
rubber and many oils and waxes, are so constituted that they are extremely useful
wherever it is desired that there shall be no electron flow.
So we see that we have plenty of raw material for Electronics. Electrons are
by far the commonest things in existence. Already we have seen that there is nothing
mysterious about the electron - that it is far simpler than a grain of sand, for
electrons are only one of the things that compose a grain of sand. If we deal with
the electron in this spirit, it will be found relatively easy to understand all
types of devices which depend on it for their operation.
Posted July 14, 2021
(updated from original post on 12/11/2014)
|











