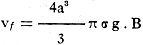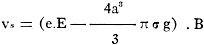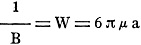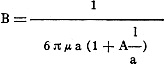|
September 1932 Radio News
 [Table
of Contents] [Table
of Contents]
Wax nostalgic about and learn from the history of early
electronics. See articles from
Radio & Television News, published 1919-1959. All copyrights hereby
acknowledged.
|
"We might say almost, that all
modern scientific investigation in the basic sciences, and a good deal of all practical
developments, are connected in some way or other with the electron." So writes Dr.
Irving J. Sax in this 1932 issue of Radio News magazine regarding
the incentive for determining as precisely as possible the mass and electrical charge
of an electron. The Bohr atomic model had just been introduced two decades earlier,
so the entire concept of particle physics was still in its infancy. As with most
areas of physics, experiments are conducted with particular biases and expectations
such that often the results are manipulated as needed to conform to preconceptions.
Look no further than the complex retrograde motion planetary models devised and
perfected by early astronomers who believed the universe revolved around the Earth.
It wasn't until a heliocentric model was accepted (following the exile and execution
of many "heretics") that orbital systems became simplified and readily explainable.
The same, to some extent, has been true of subatomic particle physics, except digging
deeper into the true nature of the beast has continually revealed greater and greater
complexity. Each time a new "fundamental" particle is discovered through empirical
testing, an new, more basic element is theorized and must be explored; to wit, the
Higgs Boson (aka "God" particle). Indeed, even the planetary-like Bohr model of
the atom was proven incorrect by the advent of quantum mechanics and the ensuing
electron orbital cloud model based on
probability
distributions (in 1932, the year of this article in fact, by Mullikan). It was
a mere five years prior to the publishing of this article that Heisenberg had formulated
his "Uncertainty Principle" that limited the precision with which a
particle's speed and mass could be determined. None of this subtracts from the importance
of each step along the way, and in fact, augments the need to continue investigating
the fundamental aspects of nature's building blocks. For the particle physics historian,
this article will prove to be a treasure trove of information.
An Interesting Experiment in Weighing the Electron and What It
May Mean

He Made the Experiment
Figure 1 - Dr. Felix Ehrenhaft, Professor of Physics at the University
of Vienna.
By Dr. Irving J. Saxl
In all our modern electronic technique, including radio technique, television,
acoustics and in all other basic sciences connected with electricity we are making
use and apply constantly the electronic theory and its fundamental unit, the electron.
We have considered the electron to be the smallest entity of matter, having one
single, definite electric charge and include it in our formula, whether we are now
building radio tubes, X-ray tubes, motor-generators or whether we are making intricate
electrical measurements. We might say almost, that all modern scientific investigation
in the basic sciences, and a good deal of all practical developments, are connected
in some way or other with the electron.
We are living in a century of electrification. New electrical machines, appliances
and instruments are still being given too humanity at high speed - and all of them
go back, in their last root, to our knowledge of the electrical phenomena and its
basis: the electron.
But what do we actually know about this tiniest entity of the universe? It is
the commonest thing in the cosmos, there is no atom of matter in which there is
not at least one electron, and yet, what is it in reality, this most important,
minutest quantity of the microcosmos?
The theory that a material body is composed of tiny "building-stones," which
cannot be divided, is not new. The Greek philosopher, Democritos, had written of
it already and even in the Hindu philosophy, first signs of this idea appear in
a general way.
Today there are a number of units which we cannot subdivide chemically. We call
them elements. From Faradays experiments on electrolysis it was found what amounts
of a certain material can be electrolytically deposited within a certain time. It
is now possible to determine the number of molecules of which the deposited substances
are composed by using the laws which have been given us by Loschmidt, making it
possible to determine, mathematically, the number of the molecules contained in
each cubic centimeter. Using this number, called "Loschmidt's Number," and using
the data taken from Faradays' laws it has been possible to determine the average
charge of a single ion. It was found to be in the order of 10-10 electrostatic
units.
Around the year 1900, Townsend, J. J. Thomson and A. H. Wilson carried on further
determinations of average values which also gave an electric charge of about 10-10
for the monovalent ion.
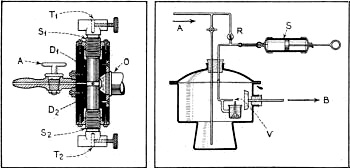
Special Condenser and Vaporizer Employed
Figure 2 - Left, shows a cross-sectional diagram of the condenser
in which the movement of the particles was analyzed. Figure 5, right, contains a
cross-sectional sketch of the vaporizer employed for producing the particles to
be "shot" into the condenser.
Of course, as far as electronics technique is concerned, the single electron
does not seem to be today of industrial importance. What is important is the effect
of a vast number of electrically-charged bodies which, after all, make important
for practical use only the average value. For going deeper into the matter, however,
it is important to know more about the individual happenings within a physically
defined body.
If we have a carload of potatoes, can we say the size of the potato is this and
that? Is it not probable that, if we observe a large-enough number we will find
small ones and large ones ... and that the small ones might be almost any size smaller
than the larger ones? Can we take the "average potato" and say: this is THE size
of the potato?
Can we, therefore, state positively that there is no smaller charge in the world?
We have subdivided the molecules and we have subdivided the atoms which, as the
name expresses, "cannot be subdivided any further." Is the electron, on the knowledge
of which we base so much of our present-day physical knowledge, is it really the
last bit of matter? Or is it just one step farther into the unknown depths of the
universe?
It was Dr. Felix Ehrenhaft, Professor of Physics at the University of Vienna
who wrote in 1909: "Smallest entities of electricity are, as far as can be predicted,
to be expected upon particles of smallest capacity" and, "These particles, however,
have to be large enough to make them just individually perceptible optically, as
it is necessary to investigate each one separately." *
For determining the size, the weight of these particles, the relation between
their electrical charge and constituting matter, the important entity e/m, , we
naturally cannot apply a chemists balance. Even the finest balances for Pregls micro-analysis
are crude in comparison with the values which have to be determined for this purpose.
Other ways have to be found for bringing about this determination.
However, a condenser balance has been developed making possible the incredible
exact weighing of body-particles smaller than the tiniest dust particles which we
see dancing in an intensive ray of sunlight if we look normally upon this projecting
ray.
The test particles which are to be investigated are brought into the field of
an electric condenser. They are strongly illuminated from the sides and are viewed
through a microscope. By putting electric charges across the condenser plates it
is then possible to move these particles up and down between the plates by electrostatic
attraction and repulsion.
From these movements of the particles in the condenser, the exact time of which
is determined, it is possible to calculate both the weight and the charge of the
body as described in greater detail at the end of this article.
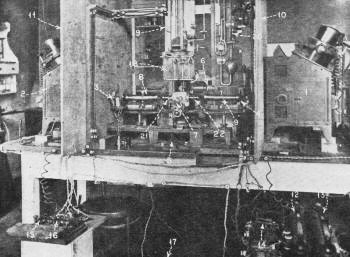
The Set-Up for Weighing the Electron
Figure - 3 This is a front view of the observer's table in the
Ehrenhaft experiment. Observation takes place through the microscope, Number 7,
the illumination of the tests particles being normally made at right angles by the
powerful projectors, Nos. 1 and 2.
Acting upon his idea, Ehrenhaft tried to take into consideration for his experiments,
particles of well-defined form and chemical characteristics. He therefore worked
with small globes of gold, platinum, silver, mercury, etc. The spherical form of
his particles he was able to show by microphotographs in white light. For fully
resolving still smaller bodies, the author has helped perfect for him the application
of ultra-violet-microphotography, following early designs of Prof. A. Koehler.†
A microscope using these principles has been manufactured by Zeiss and used in this
country for the optical analysis of alloys.
For making visible these very small particles, Ehrenhaft constructed a special
tiny condenser. It consists principally of two round plates of brass or iron of
about 1/4 inch in diameter and spaced about 1 millimeter apart. These condenser
plates form the walls of a very small air chamber (see Figure 2 which shows a schematical
cross-section through the condenser that was used in all the tests on the particles).
The two cylindrical pieces, D1 and D2, of which the condenser
is made, are screwed in from the upper and from the lower ends. By means of the
screws, S1 and S2, it is possible to correct their distance
precisely. 0 is the front lense of the microscopic objective through which the particle
is viewed. The illumination takes place, normally, upon the cross-section of the
condenser and upon the axis of the observing microscope, as shown in the close-up
of the front of Ehrenhaft's apparatus in Figure 3. The observation is made between
the condenser plates D which are embodied in a housing of Bakelite. T1
and T2 are the terminals into which the contacts of the electric conductors
are screwed securely. The gas, which is chemically and physically purified and in
which the test particles are suspended, is brought into the viewing condenser by
means of the stopcock A.
The Set-up Employed
Figure 3 shows the front view of the apparatus at the eyepiece of which one observer
is to sit. 1 and 2 are the illuminating arcs. These are high-intensity, self-regulating,
direct-current arc lights burning with about 30 amperes. It is necessary to use
2 separate sources of illumination as during the long duration of the observations
one pair of carbons may burn out. A second pair of carbons is therefore always ready
for use in the other projector so that it can be put into use immediately and make
possible a continuous observation. In addition, these two light sources put against
each other in an angle of 180 degrees are necessary also for another reason:
Most particles brought into the path of this highly intensive light react in
a specific way upon the irradiation. Dependent whether they are light-positive or
light-negative they move to the light source or run away from the source of light.
(This effect, called Photophoresis and probably in definite relation to the photo-electric
principles involved in photocells, was discovered by Ehrenhaft.)
By using two separate lighting units, diametrically opposed to each other, it
is possible, simply by illuminating from the opposite direction, to push a particle
back into the center of the observation field. This change in illumination is done
with the aid of the electro-magnetic shutters, 3 and 4, which are operated from
a double-pole switch, 5.
The particles, which have been formed in the desiccator, 6, are brought through
glass tubing into the condenser. The microscope, 7, is the device through which
the particles in the condenser are observed. The actual illumination takes place
from the left and the right. For eliminating the effect of infrared heat rays, the
light, after coming from the projectors and the shutters, passes through two filters
containing a solution in the horizontal cylinders 8 and 9. The light beam is concentrated
into the condenser field with the aid of two microscopic objectives, 21 and 22,
so that an extremely intensive "dark-field" illumination is secured. For avoiding
any indirect heat-radiation, the two projectors are placed behind the asbestos walls
10 and 11.

The Assistant's Recording Table
Figure 4 - In these intricate investigations the incorporation
of an assistant for recording is imperative. The assistant records time upon the
accurate electrically-operated stop watches, notes the applied voltages, gas pressures
and makes other important records.
Time Recording Important
The amount of voltage applied across the condenser plates can be regulated by
the rheostats 12 and 13. The switch, 14, makes it possible to change, instantaneously,
the polarity between the two plates. With a foot switch, 17, this voltage is put
onto the condenser plates.
The telegraph keys, 15 and 16, at the left lower side of the picture, operate
automatic stop-watches electromagnetically. These watches can be read down to one-fiftieth
of a second. They record the time of ascent and descent of a particle within the
condenser, the path of which is viewed upon a grid in the observing microscope 7.
This path is not a straight line, up and down, but moving in different curves. The
particle dances about following the Brownian movement; a twinkling spot upon a dark
background in the observing microscope.
The exhaust gauges, 18, consist of a series of small capillary tubes through
which the exhaust gas has to pass and by means of which it is possible to regulate
the speed of the exhaust procedure. On the manometer, 19, the air pressure in the
electric condenser can be read (through a little telescope which is on the assistants
desk). 20 is a McLeod, an instrument with which pressure (of a fraction of one millimeter)
can be read down to microns.
The observer looks into the eyepiece of the microscope so as not to loose the
path of his particle. He is kept very busily engaged in regulating the voltages
which change sometimes, especially if an accidental radioactive material has been
in the neighborhood of the condenser or other effects have taken place as to liberate
electric charges, for instance, by irradiation with ultraviolet light. The observer
has furthermore to start and stop the procedure, to regulate the exhaust, to work
the light switches so that he cannot afford the time to make actual recordings.
Mechanical recording has therefore been applied for these intricate investigations,
under the control of an assistant.
Figure 4 shows the assistant's table. It is placed behind the observer's seat,
as at the assistant's table lights are necessary. Meanwhile the entire room is kept
dark so that the observations will not be effected by any outside light. In Figure
4, 1 and 2 are the electro-magnetically-controlled stop-watches.
The actual voltage put across the condenser plates and regulated by a shunt,
is read on the precision voltmeter 3. The air pressure in the condenser is read
over a system of reflecting mirrors with the small telescope 4.
Producing the Particles
The test particles were produced by three different methods: No. 1. The first
method was to build an electric arc between two pieces of metal. This arc smelts
off minute drops of the metal to be used as particles. They evaporate from the electrodes
and coagulate in the colder atmosphere which surrounds the electrodes in the form
of balls of the dimension 10-4 to 10-5 cm. No.2. The second
method employs bodies which can be evaporated and these are brought into a small
container of glass or quartz and heated with a small flame (Mercury, Sulphur, Selenium,
etc.) to liberate a steam which condenses in the form of small balls. No. 3. Figure
5 shows the third method of producing balls of a heavy fluid as e.g. Barium-Mercury-Iodine,
Mercury, etc. These small balls will always be geometrical spheres of great accuracy
where the capillary powers are larger than the forces which bring about the "drop"
form. A vaporizer V in which specially purified gases are sucked through the opening
with the aid of a syringe-like pump, S, distributes small particles of the fluid
in which it is immersed.
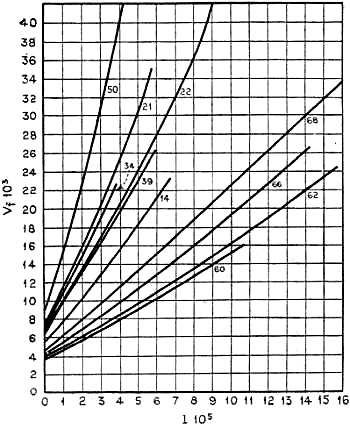
How Gas Pressure Affects Fall
Figure 6 - Diagram showing graphically the dependency of the
speed of fall of particles from the average three-mean-path of the gas as produced
at different pressures from experimental data of Dr. Max Reiss
The specific weight of this fluid is known and therefore also the specific weight
of the particles. These fall very slowly, due to their sub-microscopic size. They
are contained in the gas current and are transported together, with it, through
the funnel, B, into the condenser field.
There the particle is irradiated strongly from the side, as stated above, and
it is seen in the microscope as a luminant point upon a dark field. It is possible
to read the distance through which the particle falls freely in the air-condenser
chamber, upon a grating which is inserted into the eyepiece. If the particle carries
an electrical charge and if the plates of the condenser are charged electrically,
it is possible to move the particle upward by electrostatic power! It is also possible
to measure here by its speed!
The particle is allowed to fall again after switching off the electrical field
so that it moves downward under the influence of gravitation. This procedure is
repeated long enough so as to receive satisfactory averages of the ascent and descent
of each particle tested.
If a sphere falls in a space filled with gas, its speed of fall does not get
larger into the infinite. The friction of the sphere in the air works against gravitation
and after a certain time, the friction gets so large that the ball moves with a
constant speed. With a submicroscopic sphere as used in Ehrenhaft's experiments,
this constancy is reached after a very short time.
Investigations have shown that the velocity of such a particle is proportionate,
within certain limits, to the power acting upon it. Stating v for velocity and P
for power, we get the equation:
(1) v = P.B.
where B is a factor of proportion. Its physical meaning is the velocity under
the influence of the power which is unity. B may be called the mobility of the particle.
For a free-falling body of spherical form we have, therefore, the equation:
(2) 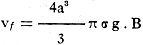
where Vf is the velocity of the fall, a is the radius, σ is
the density and g is the gravitational acceleration.
If the particle is pulled upward again under the influence of the electrical
forces put unto the condenser and has the velocity vs, then we can say:
(3) 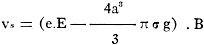
where e is the electrical charge of the particle and E is the field intensity
in the small condenser.
From equation (2) can be computed the radius of the particle:
(4) 
It is necessary to know, from other sources, the value of B, of the mobility
of the particle. This known, it is possible to determine the electric charge of
the particle, by simply inserting the value for a, from the equation (4), and the
value for E (which we can read directly on the voltmeter) in the equation (3).
The mobility B has, therefore, to be found in some other way if we want to determine
the radius and the electric charge of our particle. Stokes, the English mathematician,
calculated for the resistance W - which is reciprocal to the value of the mobility
B - which is impressed upon a sphere during its motion through a fluid:
(5) 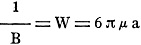
It is assumed hereby that the fluid sticks continuously to the surface of the
sphere.
For a sphere, however, which moves in a gas instead of a fluid and with reasonable
speed, too, it may not be correct to suppose that the medium will stick to the surface.
Aeromechanics and hydro mechanics have shown that this case practically never happens.
The so-called "laminar"-movement takes place only under theoretical conditions,
assuming an almost infinitely small movement of an inelastic body in a fluid of
small hydraulic mobility. Practically in all cases eddies and whirls appear which
disturb markedly Stokes' law. Experience teaches against the theory that the gas
glides along the surface of the exposed body and, in addition, the formation of
whirls takes place for gases which are so dense that the average free-mean-path
of their molecules is small against the radius of the sphere (that means sufficiently
smaller than 10-5 cm.).
It has been necessary, therefore, to correct Stokes' law. Following the calculations
of Stokes-Cunningham, this formula reads:
(6) 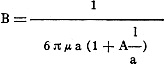
Where l is the average free-mean-path of the gaseous molecules and A is a constant
(which gives a value for the gliding and is supposed, following the theoretical
calculations, to be near unity).
Will experiments at different gas pressures especially in compressed gases, be
able to solve this problem?
Investigations at different pressures under one atmosphere have been already
made. Figure 6 shows a diagram of measurements which have been taken for each individual
particle at different pressures by Dr. Max Reiss. From these experiments which have
been made between 1 atmosphere down to about 50 mm. pressure, it seems that some
particles have a smaller density than the molecular material. On the other hand,
particles have been found, which indicate much smaller values for the supposedly
standard electric charge of an electron! It has apparently been possible to measure
single electrical charges as low as 1.10-10 electrostatic units, that
is less than one-fourth of the values found by other investigators.
For giving an idea about the incredible small forces with which we have to deal
and which we have to control experimentally in these intricate investigations, it
may be mentioned that the forces which act upon the particle are of the dimensions
of 10-10 dyne. This corresponds to an attraction with which two containers
of about one quart of water each act upon each other over a distance of about 2
miles!
If the particles with which he operated were small enough, Ehrenhaft's measurements
on individual particles showed values for the separate electronic charge which went
far below the quantum charge which is required by the theory. From other methods
the value of the electronic charge was determined to be about 4,77.10-10
electrostatic units. Dr. Robert A. Millikan in Pasadena who described the condenser
method at about the same time as Ehrenhaft††, and who was awarded
the Nobel prize found this larger charge.
In addition to having found smaller charges than the elementary quantum Ehrenhaft
states that it is not directly possible to consider the electric charges he found,
as simple multiples of the elementary charge. These conditions of being able to
build simple multiples, quanta, would be a necessary requirement, if one of the
cornerstones of modern physics should be a true natural law: the quantum theory
of Max Planck which has proven so valuable a tool for many investigations.
According to Ehrenhaft, it would be necessary to determine the value of the unit
of negative electricity much lower than 47.10-10 electrostatic units
... provided that there is existing any atom of electricity at all.
In practice we continue, today to use the electron as such in our calculations
and our engineering. But will we, under these circumstances, consider the electron
as a truly existing standard entity ... in the scientific world of tomorrow?
* Wiener Akadem. Anz. number 7 March fourth, 1909.
† Ehrenhaft & Wasser, Philosophical Magazine. Vol. 11. 1926.
†† R. A. Millikan: Physical Review XXIX, p. 260, December, 1909,
F. Ehrenhaft: Anzeiger d. Wiener Akademie d. Wissenschaften March fourth, 1909.
Posted January 27, 2022
(updated from original post on 4/15/2014)
|