|
September 19, 1966 Electronics
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early electronics.
See articles from Electronics,
published 1930 - 1988. All copyrights hereby acknowledged.
|
John Mackenzie's 1966
Electronics magazine article predicted a future where glass would
transcend its role as a passive material to become a primary semiconductor for
devices like memories, transducers, and switches. This forecast has proven
remarkably prescient. While crystalline silicon has dominated mainstream
computing, the unique properties of amorphous materials, now classified under
amorphous semiconductors or phase-change materials, have become foundational to
modern technology. The most significant realization of this prediction is in
non-volatile memory, where chalcogenide glasses are the active material in
commercial Phase-Change Memory (PCM) and the memory cells of optical discs
(CD-RW, DVD-RW). Furthermore, thin-film transistors (TFTs) made from amorphous
silicon are ubiquitous in every LCD display, and specialized glass-based sensors
are used in various applications. The author's vision of a "renaissance in glass
technology" has been fully validated, with these materials now performing the
precise "primary roles" he envisioned. Check this 2025 news headline: "Glass
Use in Semis to Triple by 2030."
Looking Through Glasses for New Active Components

John D. Mackenzie, known internationally for his work on glass,
was born in Hong Kong and educated in England. After a postdoctoral fellowship at
Princeton University, he did research at the General Electric Co. and became professor
of materials sciences at Rensselaer in 1963.
By John D. Mackenzie, Rensselaer Polytechnic Institute, Troy,
N.Y
Renaissance in glass technology is providing hundreds of new
materials - including amorphous alloys - with potential applications as low-cost
semiconductor components, transducers, memories and other devices.
Glass isn't an ordinary electronic material, even though most component designers
only use it in ordinary ways. Many kinds of components, from switching and memory
diodes to computer memories, might be cheaply produced with glass if recent discoveries
by glass scientists were exploited by electronics engineers. Glasses can now be
made as semiconductors, photoconductors, magnets, transducers, optical switches,
memory materials and superior insulators and dielectrics.
Present applications are mainly based on just a few of the obvious physical characteristics
of glass. Most engineers think of it as an easy to form, transparent, nearly impervious
solid with high electrical resistivity. So they use glass for optical parts, tube
envelopes, protective coatings, insulators and substrates. All are secondary roles,
compared with the primary roles assigned to such materials as ferrites and crystalline
semiconductors.
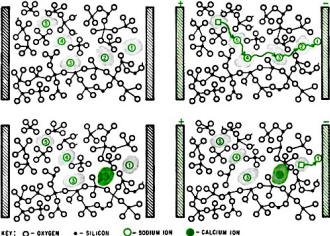
Divalent ions such as calcium improve resistivity of glass by
preventing ionic condition. Weak electrostatic bonds hold monovalent sodium ions
in place (upper left). A direct-current field, indicated by colored electrodes,
causes the ions to move between the glassy chains (upper right). The stronger bond
of a calcium ion (lower left) allows it to block the ions behind it when there is
a d-c field (lower right).
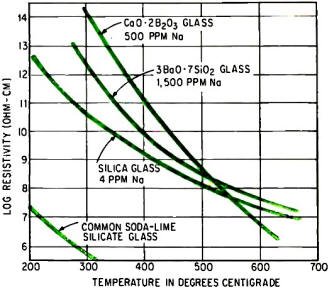
Ionic blocking of calcium or barium ions gives glasses containing
sodium much higher resistivity than silica glass, which is nearly free of sodium
but expensive.

Possible uses of semiconducting glass.
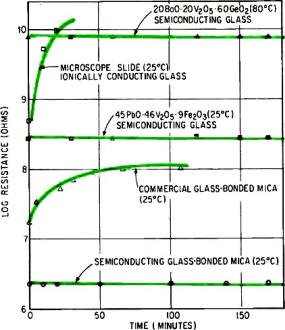
Superiority of semiconducting glass and glass-bonded mica over
ionically conducting materials is indicated by effect of 200 volts of direct current.
Increase in resistance with time indicates deterioration.
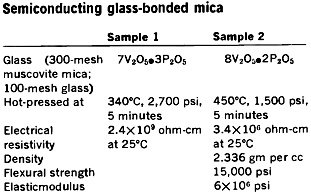
Semiconducting glass-bonded mica.
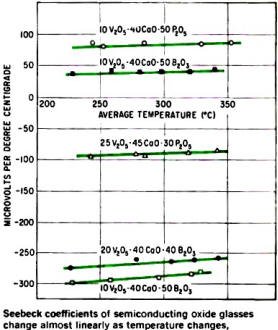
Seebeck coefficients of semiconducting oxide glasses change almost
linearly as temperature changes, indicating they would make good temperature sensors
and thermoelectric devices.

Layered structure of semiconducting glass-bonded mica, magnified
300X. Such high-strength composites can also be made with boron-nitride dielectric
and graphite.
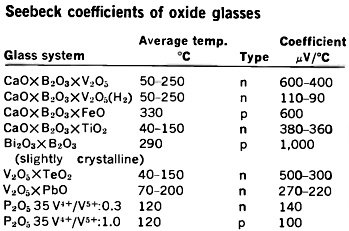
Seebeck coefficients of oxide glasses
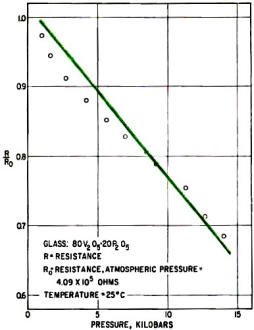
Increasing pressure linearly reduces resistivity of semiconductor
glass, probably because distances between vanadium ions decrease, allowing conduction
to increase. This glass could be used as a pressure transducer.

Semiconducting glass magnified 40,000 times by electron microscope.
"Droplets" are tiny crystals about 100 angstroms in diameter. The size and distribution
of crystals such as ferrites can be closely controlled in glass.
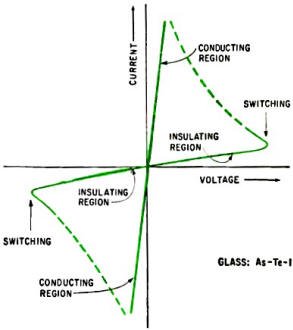
Current-voltage characteristics of diodes made of elemental arsenic-tellurium-iodine
glass. Besides pronounced switching behavior, diodes made of this semiconducting
glass will remember their states for long periods at zero bias voltage.
This general concept of glass as an auxiliary material is actually very narrow
in contrast with the capabilities of hundreds of new glasses. Few people realize,
for example, that glasses are not solids, but rigid liquids that can have an almost
infinite variety of compositions. Their room temperature resistivity can range from
only 100 ohm-centimeters to 10 °, ohm-cm. They can conduct both ionically and electronically.
Their structures can be controlled so that the glassy matrix is host to millions
of ferrite crystals, opening up the possibilities of producing microminiature but
numerically vast arrays of memory elements.
Device designers can't really be faulted for not having exploited these and other
potentially significant features of glass. Materials scientists have still not thoroughly
probed all the inherent electronic properties of glass and some of the glasses that
enhance such properties are new and untried. But it is apparent that glass is enjoying
a technological renaissance certain to make it an increasingly important electronic
device material in the coming decade. Some of the recent discoveries have already
been converted into practical hardware.
Uniqueness of Glass
If device designers are to make maximum use of glass's inherent electronic properties
they should recognize that it is unlike any other material. It can be put to work
both as a liquid and a solid because it is a "frozen-in" liquid. Like most liquids
it can - in the absence of physical disturbances and impurities - be cooled
well below its freezing point without crystallizing. The viscosity of a watery liquid
is 0.01 poise and that of glycerine is 10 poise. When the liquid becomes so cold
that its viscosity has risen to 10 poise it becomes rigid. Such a liquid is defined
as glass. Many materials can be rendered into the glassy or vitreous state in addition
to the common glasses. Since glass is a rigid liquid it never melts but only softens
when heated. This makes the fabrication of glass objects easy and permits materials
to be dissolved in glass at high temperature. The added material can then be precipitated
out in a controlled manner when the glass is cooler and rigid. This explains why
a second phase - such as ferrite crystals of controllable size - can be uniformly
distributed through the glass and why the compositions can be so varied.
The inexhaustible flexibility of composition means, of course, wide flexibility
in properties. In contrast, most solids consist of crystals. Since crystals are
stoichiometry compounds - having fixed proportions - the control over their properties
is restricted. Yet the atomic defects that make crystals so useful in electronic
applications can be duplicated in glass. Glass behaves much like crystal in its
short-range order (over spacings of a few atomic distances). How advantageous this
is, is demonstrated, for example, in the manufacture of high-power lasers. A neodymium-doped
glass laser rod 4 feet long and 3 inches in diameter is readily made by conventional
glass-making techniques. But there is no practical way of making a sufficiently
uniform single-crystal ruby laser of like size.
Glass's fabrication ease should help make practical other new electronic systems,
too. For instance, it is difficult to produce fine, superconducting magnet wire
with adequate insulation. Many years ago glass workers learned to draw hollow, hair-like
fibers of glass by pulling on a softened glass tube. Insulating superconducting
wire can now be made inexpensively by putting a slug of the superconducting metal
into a tube and softening and pulling both at once.
High-Resistivity Glasses
Formulating the glass to suppress ionic conduction improves insulating substrates,
such as those for thin-film circuits. Common oxide glasses may not be chemically
inert to the components they carry because they contain mobile and reactive alkali
metal ions, particularly sodium ions. In silicate glasses, for instance, ionic electrical
transport is entirely due to sodium ions. Their motion deteriorates circuits placed
on the glass.
Using pure silica glass, with a minimum of sodium ions, prevents deterioration.
But such glass is difficult to fabricate, is expensive and its coefficient of thermal
expansion is negligible - too low for good adherence of metal films. However, the
motions of the sodium ions can be suppressed in inexpensive silicate and borate
glasses by adding calcium and barium ions. Ionic conduction becomes negligible despite
appreciable quantities of alkalis. Some newer glasses containing calcium ions are
even much better insulators than silica, as the graph at the right indicates. One
such glass, simply fused wollanstonite, is a silicate of the composition CaO•SiO2.
Because ionic conduction occurs on an atomic scale, it has not been seen visually,
but the mechanism is probably that diagramed at the left. Apparently the sodium
ions must travel in preferential paths, which highly immobile ions such as calcium
can block to pin down the sodium ions.
Normally, the sodium ions are held in place between the silica tetrahedra (silicon
atoms bonded to four oxygen atoms) by electrostatic binding, as in the upper left
drawing. The sodium ions are cations (positively charged) while the oxygen has a
negative charge. However, when there is a d-c potential across the glass, as in
the upper right drawing, the greater attraction of the negative electrode causes
the sodium ion numbered 1 to vacate its original site. Ion 2 can then move through
the opening in the polymer-like chains of tetrahedra and replace ion 1. Ion 3 can
then replace ion 2 and so on until ion 5 has moved along the path.
Sodium ions move easily because their valence is low, only 1, and electrostatic
binding is proportional to the valence, or charge, squared. Calcium and barium are
divalent so their electrostatic binding is four times as strong. The lower set of
diagrams show how a calcium ion - the solid colored circle - stays put in a d-c
field. Sodium ion 1 can still move to the negative electrode but sodium ions 3,
4 and 5 are blocked. This blocking can occur millions of times in a piece of glass,
effectively suppressing ionic conduction and making the glass more inert and more
resistive.
Semiconducting Oxide Glasses
Most polyvalent cations are not only immobile in glass - even at temperatures
of 200° to 300° C - but because they have different valence states they make
possible electronic conduction in glass. Hence, glasses can be semiconductors, often
surprisingly good ones. Some of the possible applications for such glasses are tabulated
at the right. In a crystalline transition metal oxide like iron oxide, electronic
conduction occurs by means of a highly complex charge-transfer mechanism. The mechanism
involves carriers of low mobility, say less than 1 cm per volt-second. Electron
motion in an n-type conductor may be represented by:
Fe2+—O—Fe3+—>Fe3+—O—Fe2+
Electronic conduction can also occur in glass when enough variable valence ions
are present, since the glass's short-range order is considered similar to a crystal's.
Silicate glass containing 10% iron oxide has high resistivity but is also electronically
conducting, so it is a semiconductor.
In glasses the charge transfer is generally called "hopping" and is thought to
be similar to impurity band conduction in doped germanium. This hopping, together
with polarization induced by the charge, is called a polaron, which is now thought
to be the most likely conduction mechanism. In glasses containing vanadium oxides,
which the author has studied extensively, the hopping process is represented by:
V4+—O—V5+—>V5+—O—V4+
Many hundreds of such semiconducting glasses have been made recently. Conduction
is a bulk effect so the semiconductors are not polarized. Resistivity can vary with
the concentration of variable valence ions and other factors.
Semiconductor glasses are superior to conventional silicate glasses for all direct-current
applications. An immediate and important application is improving the long-term
performance of image-orthicon tubes. The targets of these tubes are glass disks
2 to 5 microns thick and about 2 inches in diameter. Their thickness must be uniform
and the glass resistivity should remain at about 1012 ohm-cm at room
temperature. Targets made of common glass deteriorate in time - ionic charge transfer
under the d-c potential of tube operation causes electrolysis. Targets made of the
new semiconducting glass give the tubes long life because the charge transfer is
largely by electrons, not ions, and electrolysis does not occur.
Crack-Proof Dielectrics
Semiconducting glasses can also improve glass-bonded mica, widely used in electronics
because of highly desirable dielectric and mechanical properties provided by the
mica it contains. Unlike ordinary glass, cracks apparently do not propagate through
this composite material, so it can be made into complex shapes by machining or molding.
However, charge buildup during prolonged exposure to electrons leads to dielectric
breakdown. This is due to the ionic nature of the glassy phase. If semiconducting
glass is substituted for common silicate glass, electron conduction overcomes this
difficulty, as shown at the left. Furthermore, the composite's resistivity range,
controlled mainly by the glass phase, is greatly extended.
The layered structure of the mica, as in the photomicrograph at the right, is
probably what prevents crack propagation. Many other inorganic materials also have
a layered structure. Among them are boron nitride, a good dielectric, and graphite,
a good conductor. The author formed composite glasses with both these materials.
They are machinable and possess interesting electrical properties. Varying the amount
of graphite changes the resistivity and its temperature coefficient. The glass containing
boron nitride has very low-loss factors, indicating it would be a superior capacitor
material.
Glass Transducers
Exploitation of glass semiconductors as active semiconductors is still in its
infancy. But it appears that oxide-glass semiconductors could make good temperature
and pressure transducers and that other glasses can be made into switching and active
but nonvolatile memory components.
Many oxide-glass semiconductors have large Seebeck coefficients (a measure of
thermoelectric conversion efficiency), as indicated by the typical values given
in the graph and table at the right.
The graph indicates also that the Seebeck coefficient is relatively stable over
wide ranges of temperature and that the glasses can be made as p-type semiconductors
(upper curves) or as n-type (lower curves). Thus, these glasses might make good,
low-cost temperature sensors.
Another distinction between semiconducting glasses and ionically conducting glasses
is that the electronic conductivity of semiconductors increases with physical pressure.
The resistivity change can be remarkably linear, as plotted by the author in the
graph on page 134. The pressure was applied hydrostatically at room temperature;
at 100°C, the resistance drops about an order of magnitude but is still very linear.
Analysis indicated that the conductivity increase was due to compression of the
glassy matrix, which increased the concentration of vanadium atoms per unit volume
and increased conduction by the hopping process.
An obvious potential use of this glass is as a pressure transducer. Such a transducer
could have advantages over conventional pressure sensors, since the pressure sensed
by a calibrated device could be read directly as a resistance value or used to initiate
a control signal without converting from a nonelectronic value.
Glass Ceramics
Uncontrolled crystallization in glass is undesirable because it makes the physical
properties uncontrollable. However, in recent years the controlled crystallization
of glass has become a highly successful technology with electronic implications
far wider than the present use of the technique to produce supporting materials.
Glass can crystallize because it is a metastable solid - one whose structure
may be radically changed by an impurity or physical disturbance. It tends to crystallize
at temperatures that make the ions sufficiently mobile because the crystalline phase
is invariably more stable thermodynamically.
The glass ceramics widely used in electronics for missile radomes, high-temperature
circuit boards and other components are an example of controlled crystallization.
The insulating types are detailed in a recent book. The crystals, whose formation
is triggered by electromagnetic radiation and heat, are less than a micron in diameter
and closely knit together, so mechanical properties are excellent. They can also
have high electrical resistivity and low loss factors.
Glass ceramics with active electronic properties - such as the semiconductor
glasses - can also be produced today. So it should be possible to further tailor
and enhance their electronic properties.
The composition and hence the electronic properties of such glass can be remarkably
uniform, as evidenced by the electron micrograph on page 135. The picture shows
semiconducting glass in an early stage of crystal formation, enlarged 40,000 times.
The droplets that will later become crystals are only about 100 angstroms in diameter
and the distances between them vary by only a small fraction of a micron.
No such precision can be achieved in formation of single-crystal semiconductors.
If three constituents - call them A, B and C - are combined to form a crystalline
material, A and B will probably concentrate on the surface and C will have a concentration
gradient. The electrical properties will also be nonuniform through the body of
the material, and such factors as variations in temperature coefficient of expansion
may cause strains and other defects.
Glassy semiconductors on the other hand - including the amorphous metals discussed
on page 136 - are almost like perfect single crystals in that there are no grain
boundaries, with attendant faults and concentrations of impurities. This uniformity
might someday be exploited to solve semiconductor production problems. For example,
integrated-circuit manufacturers are beginning to make monolithic circuit arrays
containing thousands of devices on a single-crystal substrate. When crystal semiconductors
are used, the number of devices ruined by random flaws in the crystals pose serious
problems. One such problem is the need to develop custom wiring patterns that avoid
the faulty devices.
New Magnetic Materials
Well on the way to laboratory success is the production of glass ceramics containing
ferrite crystals of controlled composition and distribution. This creates exciting
possibilities in development of microelectronic magnetic devices.
For example, if oxides are melted to form glass with a molecular composition
of 20% B2O3, 30% BaO and 50% Fe2O3,
controlled heat treatment will crystallize barium ferrite out of the glass. Barium
ferrite is a hard, ferromagnetic material, suitable for magnets. Virtually any ferrite
can be produced in glass hosts in this way. Thin films of barium-titanate ferroelectric
have already been made, an achievement that makes possible the manufacture of thin-film
capacitors with high dielectric constant.
From a manufacturing viewpoint, the significance of glass ferrites is the ease
of shaping and "assembling" ferrite elements, compared to the complex sequence of
processes and assembly steps needed, for instance, to produce a ferrite-core memory.
The glass can be pressed, molded, blown or drawn to produce rods, plates, films,
fibers or complex forms. The crystals can be oriented in the glass by controlling
the direction in which they grow. Phosphate glass, for one, contains long, glassy
chains; ferrite crystals will grow along these chains and their easy direction of
magnetization will be along the chains.
Selective crystallization techniques, such as selective heating or irradiation,
can control the location of the crystals. As a simple proof, the author wrapped
a resistance-heated wire around a glass cylinder. When the glass was cooled, the
ferrite appeared as a helix around the cylinder. Perhaps sense and drive wires could
be counter-wound on such a helix by an ordinary coil-winding machine to produce
a small memory. Or perhaps thin-film memories could be made with orthogonal wiring;
if in a forming stage wiring cross-points were heated by an overcurrent so the ferrite
crystallized in the underlying glass film, many of the deposition processes now
required to make planar or laminar memories could be avoided. These possibilities
remain to he proved by electronic device designers.
Optoelectronic Switches and Memories
Controlled crystallization has also led to the invention of phototropic or photochromic
glasses - materials that change color when irradiated by light of one wavelength
and then revert to their original color under light of another wavelength.
Optoelectronic system designers have worked for some time with organic phototropic
materials, but these suffer from "fatigue" - usually, after a few hundred color
changes they lose their phototropy.
Scientists at the Corning Class Works discovered that silicate glasses containing
about ½% of silver halide crystals retain their phototropy for more than
300,000 cycles." Corning is studying the feasibility of using such glasses in optical
displays, temporary data storage and data-processing systems, as well as ophthalmic,
automotive and architectural applications." It might also be used as a Q switch,
a device that boosts the peak power of laser pulses.
The phototropy is a result of glass being a rigid liquid. Silver halide is dissolved
in it at high temperature. After the glass is shaped it is heat-treated at a lower
temperature until the halide crystals are about 50 to 100 angstroms in size. The
glass is transparent to visible light, darkens under ultraviolet light and bleaches
under light of a higher wavelength or when heated. Apparently, darkening results
when the first wavelength releases halogens, but they cannot disperse in the glass
and so they recombine under the bleaching stimulus.
The reactions take from less than two minutes to hours depending on composition
and stimuli, and darkened glass eventually bleaches. Corning scientists concede
slow reactions may bar high-speed electronic applications, but point out that optical
resolution is 10 to 20 times that of photographic film. So they envision other data
and display uses."
Another phototropic glass, discovered several years ago at the Pittsburgh Plate
Glass Co., is based on glass having a short-range order like a crystal12
It has long been known that crystals can be made phototropic by generating color
centers in them. Centers can be formed in glass by melting it under highly reducing
conditions. Doping with cerium or europium ions improves the phototropy. A company
spokesman reported in August that it has not been pursuing electronic applications
of the glass because of the low volume that would be used.
Nonoxide Glasses
A group of glasses known as the chalcogenides now used for infrared transmissions
started with arsenic trisulfide in the early 1950's. The constant hunt for better
infrared transmitters that are more stable at high temperatures led to the discovery
of many such nonoxide or elemental glassy systems. Again, many possess intriguing
electronic properties.
These glasses are made by melting together various proportions of the following
elements: sulphur, selenium, tellurium, arsenic, antimony, thallium, chlorine, bromine,
iodine, phosphorus, silicon and germanium. The melt is quenched to produce the glass.
Some typical glass systems and applications are: As-S-Se, an insulator that melts
at low temperature; As-Te-I, electronic switches and memory devices; As-Se-Te, photoconductor;
and As-Ge-Si-Te, an infrared transmitter that withstands high temperature. Some
elemental glasses transmit infrared light with wavelengths as long as 20 microns.
Recently, the American Optical Co. began making arsenic trisulfide in long fibers
by pulling the melt. This allows infrared light to be piped, as in visible-light
fiber optics, and might pave the way for better methods of transmitting and steering
laser signals.
Some chalcogenides soften at temperatures below 0°C, others are viscous at 100°C,
making it feasible to dip-coat temperature-sensitive devices in glass rather than
organic materials. The softening temperatures can be controlled over a wide range.
Several years ago, Bell Telephone Laboratories reported experimental success
in dip-coating diodes with arsenic and sulfur glasses. They were good insulators
and their chemical stability was superior to organic polymeric materials.18
But according to A.D. Pearson of the research team, that work was set aside primarily
because the planar passivation process for silicon devices proved better. Nonetheless,
glass should be an attractive coating for other types of devices.
Elemental Glass Switches
Current-voltage characteristics of some elemental glasses indicate they can be
used as switching and memory devices. Pearson and his co-workers at Bell Telephone
Laboratories reported on such devices verbally in May, 1962, at an Electrochemical
Society meeting. When point contacts are made to a glass such as As-Te-I, a variation
in applied voltage switches the device from an insulating to a highly conducting
state." The switching can occur in less than a microsecond and is reversible. The
diode would remain in a given state, even under zero bias, and could remember which
state it was in for many days. A generalized current-voltage plot is shown below.
Pearson says this work, too, is inactive at present. One reason is that crystal
semiconductors have better long-term stability and thus are considered more suitable
for telephone system applications. However, several companies in the U.S. and Europe
are actively attempting to develop practical uses for elemental glasses. Little
of this work is reported in the open literature since it is considered proprietary.
[The Energy Conversion Devices Co. disclosed this month that the semiconductor
devices it has begun offering commercially are principally elemental glasses and
amorphous alloys. The active materials are homogenous, without p-n junctions. Devices
being made or under development include a-c and d-c switches, temperature and pressure
transducers, adaptive devices and nonvolatile memory elements. - Editor]
Photoconducting Glasses
The Russians have extensively studied the electronic properties of nonoxide glasses.
One of the better known properties is photoconductivity - the photoconductivity
of glassy selenium, for example, makes it a basic material for xerography.
Glasses based on selenium, tellurium and arsenic are also photoconductors.16
In contrast to crystalline materials, the conductivity of the glasses is insensitive
to impurity contents. This means electronic properties are easier to control during
manufacture. Silicon and germanium are rendered into the amorphous - or glassy -
state by vacuum evaporation and show higher conductivity and other interesting variations
in semiconducting property.' It is not known for certain whether such vapor-deposited
noncrystalline films are glasses. Glasses are usually considered materials made
by cooling molten materials.
Still another new form of glass of interest to electronics is electronically
conducting glass based upon mixtures of oxides and nonoxides."
Quenched and Pseudo Glasses
Quenching and other exotic techniques of producing glasses from metals and other
unusual materials are also being tried. In one quenching technique, called splat-cooling,
droplets of molten metal are sprayed at high speed onto a revolving substrate cooled
by liquid nitrogen. Alloys such as silicon and gold or silver can be made as glass
by this method,18 and some metals that normally will not mix can be rendered
into glassy or amorphous alloys.
The technique is based on the fact that undercooled liquids can be prevented
from crystallizing if the cooling - called quenching - is rapid enough. Since quenching
rate depends upon heat conduction, the smaller the amount of material, the easier
it is to make it into glass. Tiny glass bodies may not interest window and bottle
manufacturers, but small amounts of materials can be highly important in processes
such as those used to produce semiconductor integrated circuits.
Although little information is available on metal glasses, related materials
give strong indications that interesting electronic properties can be expected.
These indications come from noncrystalline films formed by vapor deposition. The
films are not necessarily similar to quenched glass films of the material, but often
they are alike." Silicon dioxide films formed by diverse techniques - such as melting,
vapor-phase hydrolysis, sputtering, thermal decomposition and shock-wave transformation
and neutron bombardment of crystals - have similar refractive indexes and infrared
absorption bands. One can conclude that each SiO2 sample is glass.
If one also assumes that all vapor-formed, noncrystalline films are glass, many
new electronic glasses or pseudo glasses have been discovered in recent years. Among
these are: bismuth, a superconductor; MgO and MgF2, infrared transmitters;
and cobalt-gold alloys, ferromagnetics. The latter should settle the question whether
a noncrystalline material can be ferromagnetic.21
The cobalt-gold alloys were made by researchers at the International Business
Machine Co. They vacuum-deposited the two metals onto a liquid nitrogen cooled substrate.
The material remains amorphous until heated. As it becomes crystalline, its coercive
force jumps, typically from 20 to 40 oersteds. The alloy appears to have a magnetic
structure like fine-grained Permalloy.
It seems, therefore, that microelectronic systems designers searching for new
magnetic materials now have a possible alternative to conventional materials and
oxide glasses containing ferrite.
References
1. J.D. Mackenzie, "Vitreous State," Encyclopedia of Physics, Reinhold Publishing
Corp., N.Y., 1966, p. 769.
2. S.M. Cox, "Ion migration in glass substrates for electronic components. Physics
and Chemistry of Glasses 5, 1964, p. 161.
3. J.D. Mackenzie, "Fine Structure in Glass from Ionic Transport and Volumetric
Considerations," Proc. VII International Congress on Glass, 1965, p. 22.
4. J.D. Mackenzie, "Semiconducting Oxide Glasses," Modern Aspects of the Vitreous
State, Vol. 3, Butterworth Inc., Washington, 1964.
5. J.D. Mackenzie and S.P. Mitoff, "Semiconducting Glass," U.S. Patent No. 3,258,434,
June 28, 1966.
6. J.D. Mackenzie, "Semiconducting Glass-Bonded Mica - A New Electronic Ceramic
Composite," Bulletin American Ceramic Society, 45, 1966, p. 539.
7. P.W. McMillan, "Glass-Ceramics," Academic Press, N.Y., 1964.
8. H. Tanigawa and H. Tanaka, "Studies on Magnetic Microcrystalline Materials
Produced by Crystallization of Glasses in the System 6203-BaO-Fe2O3," Bulletin,
Osaka Government Industrial Research Institute, Japan, 15, 1964, p. 285.
9. A. Herczog and S.D. Stookey, "Glass and Methods of devitrifying same and making
a capacitor therefrom," U.S. Patent No. 3,195,030, July 13, 1965.
10. W.H. Armistead and S.D. Stookey, "Photochromic Silicate Glasses Sensitized
by Silver Halides," Science, 144, 1964, p. 150.
11. G.K. Megla, "Optical Properties and Applications of Photochromic Glass,"
Applied Optics, 5, 1966, p. 945.
12. E.L. Swarts and J. Pressau, "Phototropy of Reduced Silicate Glasses Containing
the 575 m Color Center," Bulletin American Ceramic Society, 42, 1963, p. 231.
13. A.D. Pearson, "Sulphide, Selenide and Telluride Glasses," Modern Aspects
of the Vitreous State, Vol. 3, Butterworth Inc., Washington, 1964.
14. A.D. Pearson, W.R. Northover, J.F. Dewald, and W.F. Peck, Jr., "Chemical,
Physical, and Electrical Properties of Some Unusual Inorganic Glasses," Advances
in Glass Technology, Plenum Press, N.Y., 1962, p. 357.
15. T.N. Vengel and B.T. Kolomiets, "Vitreous Semiconductors," Soviet Physics
- Technical Physics, 2, 1957, p. 2,314.
16. J. Stuke, "Electrical and Optical Properties of Elementary Amorphous Semiconductors,"
Conference on Electronic Processes in Low-Mobility Solids, Sheffield University,
England, 1966.
17. B.T. Kolomiets, "Vitreous Semiconductors," Physics Status Solidi, 7, 1964,
p. 713.
18. W. Klement, Jr., R.H. Willens, and P. Duwez, "Noncrystalline Structures in
Solidified Gold-Silicon Alloys," Nature, 187, 1960, p. 869.
19. D.R. Secrist and J.D. Mackenzie, "Identification of Uncommon Noncrystalline
Solids as Glasses," Journal American Ceramic Society, 48, 1965, p. 487.
20. D.R. Secrist and J.D. Mackenzie, "Preparation of Noncrystalline Solids by
Uncommon Methods," Modern Aspects of the Vitreous State, Vol. 3, Butterworth Inc.,
Washington, 1964.
21. S. Mader and A.S. Nowick, "Metastable Co-Au Alloys: Example of an Amorphous
Ferromagnet," Applied Physics Letters, 7. 1965, p. 57
|







