|
April 1973 Popular Electronics
 Table of Contents Table of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
 According to Samuel Milbourne's "Battery
Types and Their Characteristics" article in Popular Electronics
magazine, in 1973 there
were about 400 different battery types to choose from when deciding what to
buy for your automobile, electronic device, uninterruptible power supply, flashlight,
etc. I don't know what the number of types is today, but it must be in the
thousands. Nominal voltage, case size and shape, energy capacity (amp-hour rating),
current delivery capacity ("C" rating), environmental accommodation, connection
type (contact, solder, screw-on, or push-on terminals), chemistry, number of recharge
cycles (for secondary batteries), and a host of other choices are available nowadays.
Every time I need to order a new Li-Po battery pack for a model airplane or helicopter,
I spend quite a bit of time searching through mAh versus weight and physical size
specifications to identify the best - and most affordable - option. There will
never be a one-size-fits-all battery. If you are interested in
vintage batteries,
then you'll surely like Eric Wrobbel's webpage. See also "Inside
the Dry Cell" from the May issue of 1963 Radio-Electronics. According to Samuel Milbourne's "Battery
Types and Their Characteristics" article in Popular Electronics
magazine, in 1973 there
were about 400 different battery types to choose from when deciding what to
buy for your automobile, electronic device, uninterruptible power supply, flashlight,
etc. I don't know what the number of types is today, but it must be in the
thousands. Nominal voltage, case size and shape, energy capacity (amp-hour rating),
current delivery capacity ("C" rating), environmental accommodation, connection
type (contact, solder, screw-on, or push-on terminals), chemistry, number of recharge
cycles (for secondary batteries), and a host of other choices are available nowadays.
Every time I need to order a new Li-Po battery pack for a model airplane or helicopter,
I spend quite a bit of time searching through mAh versus weight and physical size
specifications to identify the best - and most affordable - option. There will
never be a one-size-fits-all battery. If you are interested in
vintage batteries,
then you'll surely like Eric Wrobbel's webpage. See also "Inside
the Dry Cell" from the May issue of 1963 Radio-Electronics.
What's That Battery Number the Same As?
 By Samuel C. Milbourne By Samuel C. Milbourne
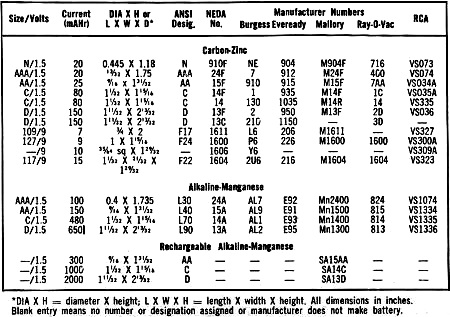
A certain amount of confusion exists with regard to battery types and numbers.
Consequently, it is handy to have a chart that gives the specifications of the various
batteries commonly used in such consumer equipment as transistor radios, toys, clocks,
etc. Our table is limited to listings for 1.5- and 9-volt batteries. Since there
are more than 400 battery types and thousands of manufacturers' numbers from which
to choose, we make no pretense that the table is complete. However, the listings
should cover most needs.
You will note that the batteries are divided
into carbon-zinc, alkaline-manganese, and rechargeable alkaline-manganese types.
They are listed in the order of their current delivery capabilities from least
to greatest. Too, each common battery type is supplied with such statistics as:
size, American National Standards Institute (ANSI) and National Electronic Distributors
Association (NEDA) numbers, etc.
In general, batteries of the same physical size and shape with the same voltage
output and terminations can be substituted for each other so long as the maximum
currents drawn by the circuits can be accommodated by the substitutes. So, you can
put an alkaline type in place of a zinc-carbon battery, and a rechargeable alkaline
in place of a regular alkaline battery.
It is interesting to note that the current delivery of the more recent alkaline
batteries is several times greater than the older carbon-zinc batteries. True, the
initial cost of the newer batteries is much greater, but they do not need to be
replaced nearly as often as carbon-zinc cells, and if the alkalines are rechargeable
types (with higher prices than the regular type alkalines), they can be renewed
several dozen times before replacement is needed.
Upon perusing our table, you will note that many categories of batteries are
not listed. These include nickel-cadmium, mercuric-oxide, silver-oxide, and lead-acid
types. These have not been included in the listing because of their much higher
costs and the future likelihood of losing out, at least to some degree, to the rechargeable
alkaline types.
*DIA X H = diameter X height; L X W X H = length X width X height. All dimensions
in inches. Blank entry means no number or designation assigned or manufacturer does
not make battery.
We will not go into the subject of recharging batteries here since this topic
has been amply covered in other articles. But you should bear in mind the following
facts that apply to all types of batteries: First, do not allow batteries to run
completely down; test them often under adequate loads. Second, do not attempt to
recharge a "leaky" or "rusted" battery; the electrolyte is corrosive and will ruin
charger contacts. And, third, do not recharge batteries at abnormally high currents;
the batteries will heat up and may explode.
Testing a battery requires a resistive load that reflects the allowable battery
discharge current and an accurate dc voltmeter. To illustrate, a Burgess No. 2 or
Eveready No. 950 battery can accommodate a 150-mA load across its 1.5-volt poles.
By using Ohm's Law (R = E/I) you can determine that the proper test load for these
batteries would be 10 ohms (1.5/0.15 = 10). The resistor selected for the test can
be rated at 1/2 watt. To make the test, place the load resistor across the battery's
terminals or poles and connect the meter's leads, in proper polarity, across the
resistor. The meter will then indicate between 1.5 and 0.9 volts if the battery's
charge has not been irretrievably depleted. If you obtain a meter indication of
less than 0.9 volt, discard the battery and replace it with a new unit.
Batteries should be recharged or replaced long before they are completely exhausted.
It is not harmful to recharge a 1.5-volt cell before its charge drops to 0.9 volt.
In fact, it is beneficial in that it provides longer total life from the battery.
Posted March 22, 2023
(updated from original post
on 10/10/2017)
|










