|
February 1973 Popular Electronics
 Table of Contents Table of Contents
Wax nostalgic about and learn from the history of early electronics. See articles
from
Popular Electronics,
published October 1954 - April 1985. All copyrights are hereby acknowledged.
|
Around the time this
article was written, the first wave of the solar and wind (which essentially is
also solar) power generation craze was settling in. A few small windmill generators
popped up around where I lived in the Annapolis, Maryland area, but they were mostly
owned by hippie Earth worshipers who eschewed modern conveniences and didn't need
hot water for bathing anyway. Most of that generation (pun intended) of windmills
put out direct current (DC - typically 12 or 24 volts) rather than tying in with
the AC line power, and required separate electric wiring in the house. People used
appliances and light fixtures designed for recreational vehicles. We knew a very
nice older man and his wife who lived "off the grid" and grew most of their produce
and even kept a goat for milk (they were clean people). They had some photovoltaic
(PV) solar cells to supplement the windmill.

Solarex Corporation Building Frederick, Maryland
On a larger and more serious scale, in the 1970s a company named Solarex Corporation
that intended to manufacture PV cells on a commercial basis, constructed a facility
in Frederick, Maryland. It had a distinctly futuristic look, and turned a lot of
heads of drivers cruising along I-270, north of Washington, D.C. I drove past it
every workday for three years in the early 1990s when commuting between my home
in Hagerstown, MD, and COMSAT in Germantown, MD. By then it had been largely abandoned.
The fad did not last long due to low efficiency and high cost of production. Government
was not heavily subsidizing the industry like it does now, so the industry could
not support itself with sales profits.
See also "Silicon
Solar Cells" in the November 1973 and "Solar Battery" in the
October 1954 issues of Popular Electronics. RF Cafe visitor Joe Cahak wrote
an very nice article titled, "Solace in Solar," on the solar
cell array that was installed on his house.
Proposed Solar Energy Station Capable of Generating 1000 Megawatts
of Power for Small Cities
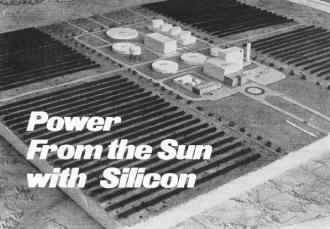
Scale model of 1,000-megawatt power station proposed by researchers
at University of Arizona. Parallel dark lines represent three square miles of solar
energy collectors.
By David L. Heiserman
Small children sometimes dream of catching a beam of sunlight in a box and releasing
it to light their rooms at night. Sophisticated adults might scoff at this notion,
but scientists and inventors have been working seriously on similar kinds of ideas
since the beginning of the industrial revolution. The dream has been to capture
some of the heat energy abundant in desert sunlight, store it in some kind of container,
and release it at a controlled rate to operate machinery, generate electrical power,
heat buildings, or distill sea water.
Until recently, solar energy conversion and storage schemes have been too complicated
or inefficient for large-scale applications. It is one thing to build a solar furnace
that will cook food and distill sea water for a single family and quite another
to scoop up enough energy to meet the demands of a modern city. Dr. Aden Mienel,
Marjorie Mienel, and Dr. Bernard Seraphin at the University of Arizona believe that
they have the answer. They have already proposed construction of a solar energy
station capable of generating 1000 megawatts of electrical power for small cities
near the Mojave Desert.
The scheme employs a new kind of device that can be described as a "solar energy
trap" - plates of thin-film materials that let solar energy enter a sealed chamber
but will not let it out again. The device works surprisingly like the child's idea
of capturing sunlight and releasing it at a later time. The only real difference
is that the modern solar collector converts the incoming sunlight into heat energy,
retaining all heat that might try to escape in the form of infrared heat energy.

Fig. 1. The basic solar energy collector- consists of evacuated
glass envelope with sheets of selective thin films running through its center. Thicknesses
of films have been greatly exaggerated. The silicon thin film absorbs solar radiation,
converting it into heat energy. Since silicon is a poor radiator of infrared heat
energy, the heat is trapped in bottom half of collector. Gold film improves the
efficiency of device by acting as a perfect mirror to infrared. Channel of molten
sodium metal carries away collected heat at the high temperature of about 1000°F.

Fig. 2 Molten sodium metal, heated to 1000°F. in solar collectors,
transfers most of its energy to thermal storage tank full of molten sodium and magnesium
salts. Water passing through storage tank gathers up some of energy and emerges
as superheated steam for turbines and electrical power generators. Thermal storage
tank is large enough to supply superheated steam throughout the night as well as
for several cloudy days at a time.
Besides being a source of heat for generating electrical power, this scheme should
interest people working in electronics because it takes advantage of some new ideas
and processes developed especially for the electronics industry. To understand exactly
how the new solar collector works, electronics specialists might have to brush up
on their knowledge of heat absorption and radiation.
How It Works
The common base materials for semiconductors (silicon, germanium, and gallium)
have some special optical, as well as electrical, properties. These materials appear
opaque to light in the visible part of the spectrum but quite transparent to the
infrared wavelengths. Laser diodes and light-emitting diodes demonstrate this fact
quite clearly. Since the human eye is tuned to the visible part of the spectrum,
crystals of silicon have an opaque, blackish-gray appearance. Infrared light generated
deep within these crystals, however, emerges from them as if the crystals were totally
transparent. The semiconductor base materials, then, behave like selective optical
filters; they are opaque to visible light in the 0.5-micron region but transparent
to light in the 1.5-micron region of the spectrum.
Whenever visible sunlight strikes any dark-colored or opaque material, the latter
absorbs most of the energy and converts it into heat. The material can dissipate
this absorbed energy by way of convection currents in the air, heat conduction to
other materials in close contact with it, or by infrared heat radiation. Under ordinary
conditions, a sample of silicon placed in bright sunlight tends to get rather warm.
Air convection currents and heat conduction carry away most of the absorbed energy,
preventing the silicon sample from becoming unusually hot. However, if the silicon
were suspended inside an evacuated glass container, these two cooling mechanisms
would be defeated, and the only way the silicon could dissipate its absorbed energy
is by infrared heat radiation.
Since silicon is a notably poor conductor of infrared heat energy, suspending
it in a vacuum and placing it in intense desert sunlight makes it grow extremely
hot in a very short time. Without resorting to any kind of lenses or parabolic mirrors,
researchers at the University of Arizona have used thin films of silicon to produce
temperatures in excess of 1000°F.
Applying the Film Concept. The basic "solar energy trap" consists of a thin film
of silicon deposited onto one side of a 6"-wide sheet of some transparent substrate
material. A thin film of gold, deposited on the other side of the substrate, enhances
the efficiency of the device by acting as a mirror to trap infrared radiation. The
substrate and its films fit in the center of an evacuated glass cylinder (see Fig.
1). The entire assembly, along with thousands of others like it, are put into an
elaborate collector array in which the silicon films face the sky.
Solar energy striking the silicon films change into heat energy. Since the optical
properties of the silicon prevent infrared radiation from returning to the sky,
the absorbed heat builds up in the films and in the substrate material.
A channel of sodium metal, running along the underside of the substrate, absorbs
much of the heat energy by conduction. When the temperature gets high enough, the
sodium melts. An elaborate system of series and parallel high-temperature plumbing
interconnects the sodium channels in all solar energy collectors. By pumping the
molten sodium through the solar collectors, it is possible to collect and store
enough thermal energy to operate a steam turbine power plant on a 24-hour basis.
Proposed Solar Power Plant. The solar energy system proposed by the University
of Arizona consists of three basic sections - an energy collection system, a thermal
storage system, and a power generating system. The collection system includes approximately
3 sq mi of collector arrays and interconnecting plumbing. The thermal storage system
is a 300,000-barrel tank of sodium and magnesium salts buried just beneath the surface
of the earth. The power generating system is a conventional 1000-megawatt steam
turbine station.
Molten sodium, leaving the collector arrays at a temperature of about 1000°F,
gives up most of its thermal energy as it circulates through the storage tank. Pumps
return the molten sodium, now cooled to about 200°F, to the collector array.
Another closed system, this one containing pure water, retrieves thermal energy
from the storage tank in the form of super-heated steam. At a pressure of 1200 psi
and a temperature of about 1000°F, the steam operates a set of conventional
power-generating turbines. After dropping most of its energy at the turbines, the
steam cools and condenses to a liquid state before returning to the thermal storage
tank at a temperature of about 212°F (see Fig. 2).
The University of Arizona researchers believe that the overall efficiency of
the system will be close to 30 percent. Considering that the "fuel" costs absolutely
nothing, the system promises to become the most inexpensive source of large amounts
of power man has ever devised.
While the new system has some of the ecological disadvantages that plague other
so-called "clean" power sources (in this case, thermal pollution), the problem can
be minimized. To counter thermal pollution, researchers plan to use the excess heat
to distill sea water. Rough calculations show that the 1000-megawatt solar energy
station could produce as much as 50-million gallons of fresh water daily. This is
enough fresh water to cause some people to think seriously about using areas around
the desert power plants for raising crops and other agricultural purposes.
Posted May 7, 2020
|











