|
June 1963 Radio-Electronics
 [Table of Contents] [Table of Contents]
Wax nostalgic about and learn from the history of early electronics.
See articles from Radio-Electronics,
published 1930-1988. All copyrights hereby acknowledged.
|
This "Which Dry Battery for
You" article is a follow-on from the previous month's "Dry Cell Battery Types"
in Radio-Electronics magazine. It was a time long before the dominance
of rechargeable lithium batteries. In 1963, battery-powered devices were nowhere
near as widespread and diverse as they are nowadays. Hand tools like drills, saws,
routers, planers, and screwdrivers got their power either from a wall outlet or
the user's arm and hand muscles. Lawn mowers, grass and hedge trimmers, chain saws,
and snow blowers were powered mostly by gasoline, although some models plugged into
the wall. Those devices which did use batteries most often had no built-in recharging
capability, and most cells were of the primary type and therefore were not rechargable.
The most prevalent type of rechargeable (secondary) battery was
nickel cadmium (NiCad).
NiCad cells have a lower energy storage density than today's more common nickel
metal hydride (NiMH) and lithium-ion (LiIon) or lithium polymer (LiPoly), and earlier
NiCads exhibited a significant "memory" characteristic which caused them to lose
capacity if left on a "trickle charge" or were constantly recharged after only a
partial discharge.
Which Dry Battery for You?

There is a best dry battery for every job. This article will help you pick it
out.
By Gordon E. Kaye*
Dry batteries are made in three common types, commonly called zinc-carbon, alkaline
and mercury. The zinc-carbon battery is further divided into four varieties. These
were described in the article "What Is A Dry Battery?" in the May, 1963, issue.
Each of these types has its own best applications, due to its composition or the
proportions of the elements used in its mix. The table illustrates some typical
consumer applications and the reasons for choosing correct battery types for them.
There are hundreds of other industrial, military and commercial devices using dry
batteries.
A rather special application is that of voltage standard. The industrial-grade
mercury battery may be used as a voltage-reference source. (Some varieties of mercury
cells made with a manganese dioxide blend are not suitable as a voltage reference.
The MnO2 causes a reading of 1.4 volts. This can be spotted easily.)
At intermittent drains up to 1 ma, it is within 1% of its original 1.357 volts for
a period of 2 to 10 years. Aged cells, after 3 years, can have a long-term stability
of 0.1 %.
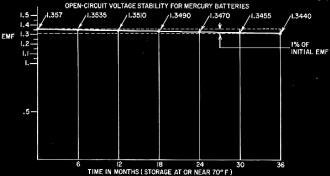
Fig. 1 - Chart shows excellent shelf-life of mercury cells
and batteries.
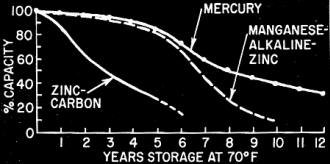
Fig. 2 - Expected shelf-life of the three battery systems
at 70° F. Storage at 120° F reduces shelf-life to one-fourth the values
shown.
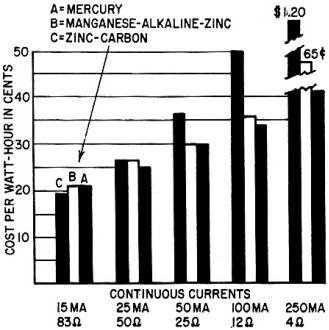
Fig. 3 - Comparative costs per watt-hour of the three battery
systems at different load levels.
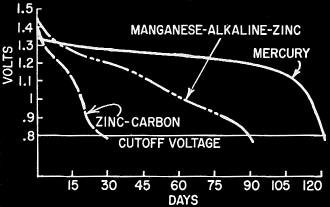
Fig. 4 - Life-span of cells discharged into 60-ohm loads
for 8 hours per day at 70° F.
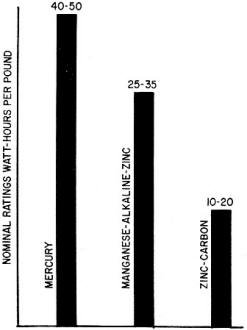
Fig. 5 - Comparative watt-hour per pound ratings of the three
primary cell systems.
Direct measurements may be made on these cells with ordinary voltmeters. Voltage
potentiometers are not needed, except where more precise readings are required and
calibration against a primary standard is called for. You can attain short-term
accuracies in the order of one part in a million, especially if the temperature
is a stable 120°F, and the cell has been aged. The average open-circuit voltage
of these cells doesn't seem to drift over the years, as shown in Fig. 1.
Selecting a Battery Type
The simplest economic viewpoint in dry-battery use is the cost of delivered energy
per hour (cost per watt-hour). Not so obvious is the inclusion of a shelf-life factor
(Fig. 2) as well as a quality-rating factor. The latter would be important in high-quality
appliances such as battery-operated tape recorders, cameras, wristwatches or light
meters. If equipment is left unused for a long time, leakage and loss of capacity
can raise battery operating costs. Equipment damage and undelivered energy are valid
charges against a cell system.
The chart in Fig. 3 compares the cost per watt-hour for the AA penlight cell
in the three dry-cell types. Various current rates are shown against costs, based
on list prices for top-grade cells. It can be seen that heavy loads raise energy
costs appreciably. There is an economical cell size for each application. Also,
the alkaline systems are less costly in heavy-duty, long continuous service, especially
where voltage levels are to remain high (Fig. 4). Shelf life and leakage factors
also tend to favor these systems.
Zinc-carbon cells and batteries are more economical initially, and are favorable
in lightly loaded, intermittent applications. They are less costly in the larger
cell sizes due to a higher efficiency when operating at nominal rates.
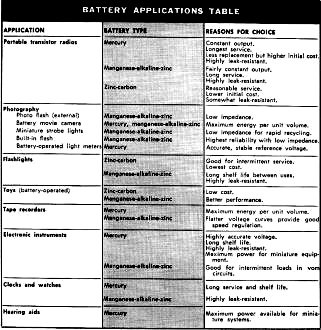
Battery Application Table
Watt-hour ratings per pound (Fig. 5) are decisive in many applications where
weight and bulk must be kept to a minimum. The dry-battery products manufactured
today represent the accumulation of 70 years of industrial electrochemical experience,
beginning with Leclanché and continuing right through to the advanced-design
mercury cell. The number of batteries required per product is reduced, battery efficiencies
are higher, and their operating costs are consequently lowered. An example of this
is seen in the compact, modern transistor radio.
A 10.8-volt voltage-reference battery.
Selecting the right battery, how-ever, requires that you consider battery capabilities
in terms of application requirements. Regardless of the application, there is a
correct cell or battery design for maximum performance. The table and charts will,
we hope, assist you in your selection.
* Application engineer. Mallory Battery Co.
|