
Above is a scary sight - a severely bloated Li-Po battery pack (next to an unbloated
one). These two packs arrived in the same undamaged shipping container, but one looked like it was ready
to explode - Scary! I took the photo and then put the pack outside in case it decided to self-destruct.
Lightweight lithium ion polymer (Li-Poly) batteries have made a huge impact on the performance, and
subsequently the acceptance of electric systems as a replacement for the traditional nitromethane (nitro)
and gasoline power systems in model airplanes and helicopters. Their energy density (Wh/kg is the most
common unit of measure), combined with the relatively new and extremely powerful brushless motors, electric
power systems are rivaling the internal combustion systems in terms of both energy and duration.
Development of both the brushless motors and the advanced battery technologies has been, both officially
and unofficially, a joint venture between government and civilian research and development efforts.
If you keep up with the news headlines for NASA, defense contractors, green energy researchers, and
similar organizations, you have witnessed the plethora of new vehicles that have been built tested,
and in may cased deployed in the field. These range from micro air vehicles that carry surveillance
equipment for the military, to hybrid and fully electric passenger vehicles, vastly improved power sources
for portable devices and equipment, and a whole lot of new products for the model enthusiast.
 To the
left are two Li-Poly battery packs (red) in my scale model of the
Piper J-3 Cub (covered in the L-4
color scheme). Each pack contains two, 3.7 V Li-Poly cells wired in series internally (7.4 V for the
pack). Externally, they are plugged into a "Y" harness to double the energy rating from 1,500 mAh to
3,000 mAh. This particular model originally used an OS 0.25 in³ nitro motor, and was converted to electric
power using a geared, brushed motor and these batteries. Total flying weight increased by only a couple
ounces. To the
left are two Li-Poly battery packs (red) in my scale model of the
Piper J-3 Cub (covered in the L-4
color scheme). Each pack contains two, 3.7 V Li-Poly cells wired in series internally (7.4 V for the
pack). Externally, they are plugged into a "Y" harness to double the energy rating from 1,500 mAh to
3,000 mAh. This particular model originally used an OS 0.25 in³ nitro motor, and was converted to electric
power using a geared, brushed motor and these batteries. Total flying weight increased by only a couple
ounces.
Events tailored specifically to electric-powered models have been created at the major contests worldwide,
and some "electrics" are taking top prize positions in events where internal combustion engines are
used in the same events as electrics. Energy densities of four to eight times as great as the older
nickel metal hydride (NiMH) and nickel cadmium (NiCad) cells have been realized. Lithium technology
has markedly improved flight performance and duration even with maintaining the existing brushed
motors in the airplanes and helicopters. Some reports indicate that the use of electric power systems
in model aircraft now exceeds the use of nitro and gasoline power. Model boat and car enthusiasts were
easier converts because weight concerns are not as great.
 Melanie holding my
Herr Engineering
J-3 (right) covered in the U.S. Navy NE-1 color and
trim scheme. Melanie holding my
Herr Engineering
J-3 (right) covered in the U.S. Navy NE-1 color and
trim scheme.
One reason that electric power has gained so much momentum is the huge number of battery powered
models that can be found in retail stores like Wal-Mart, Target, and Radio Shack,
and of course online from Amazon. The
Cox and Testors
control line models of yesteryear using glow fuel (nitro) and the little 0.049 in³ internal combustion
engines that once lined the shelves have given way to dozens of free flight and radio controlled airplanes
and helicopters made of Styrofoam and come ready to fly out of the box - just charge and toss them into
the air. Success is virtually guaranteed. For $30, you can buy a remote control helicopter that is more
controllable by the novice flyer than the radio controlled helicopters I had back in the 1970s and even
the 1990s. Many more varieties of electric powered airplanes and helicopter models (with differing degrees
of complexity and cost) can be purchased at hobby shops and through online distributors.
 Coaxial rotor remote control helicopters
come ready to fly. This one features microprocessor controlled stabilization and
even an onboard wireless camera. Price: A mere $40. Coaxial rotor remote control helicopters
come ready to fly. This one features microprocessor controlled stabilization and
even an onboard wireless camera. Price: A mere $40.
Another motivation for the adoption of electric power is the nearly unperceivable noise emitted by
them. As areas become more and more densely populated, finding and keeping an adequate flying site is
increasingly difficult. Noise ordinances and nearby residents unwilling to tolerate the noise of the
internal combustion engines have run off many modelers who used to enjoy being able to fly their craft.
Now, with the "quite revolution" firmly in place, nobody even knows that you are flying unless they
happen to see your plane in the air. The mostly irrational fear associated by the ignorant with the
noise emanating from a nitro engine is utterly assuaged by the lack of noise coming from an electric.
I say mostly irrational because in fact even the electric powered models can inflict a substantial amount
of harm to a body or an obstacle upon contact. You can bet that I will not be to one to enlighten anyone
(other than you, dear reader) as to the remaining potential for damage. Ignorance on the part of the
layman is bliss on the part of a modeler desperate for a convenient place to fly.
 This
image shows the totality of the electronics from my Air Hog. Main rotor shaft is in upper left corner,
battery (probably about 25 mAh) in lower right. This
image shows the totality of the electronics from my Air Hog. Main rotor shaft is in upper left corner,
battery (probably about 25 mAh) in lower right.
As a side note, I will point out that responsible fliers of models are
members of organizations like the Academy of Model Aeronautics (AMA), and
therefore carry accident liability insurance. In the case of the AMA, I am
covered for up to $2,000,000 (that's right two-million dollars) per incident.
Another side note: Back in the early 1970s, whilst still a pup (about 14 years
old), I managed to "land" one of my RC airplanes (an Andrews S-Ray, for those of
you in-the-know) inside the local elementary school library one weekend. My
radio system (on 27.195 MHz) and piloting skills were very bad at the time, and
when combined with a gust of wind, my craft broke through the glass window. Even
in my freaked-out mood, I found the courage to ride my bicycle over the
principal's house and report the incident, and the AMA covered the cost of
repair.
OK, now that the very long and some might say unnecessary introduction to Li-Po batteries is out
of the way, let us get down to the business of presenting some of the relevant technical facts.
 Here is an "outrunner" brushless motor by E-flight, model 450,
rated at 890kV (890 rpm/V), installed in an electric-powered radio controlled sailplane (note the folding
propeller). It is a slotted 12-pole motor, and draws 14 A of current at full load. It uses an electronic
speed controller that output a 3-phase voltage. Although commonly referred to as a DC motor, it really
is a miniature 3-phase AC permanent magnet synchronous motor, although induction types are sometimes
used in other applications. Here is an "outrunner" brushless motor by E-flight, model 450,
rated at 890kV (890 rpm/V), installed in an electric-powered radio controlled sailplane (note the folding
propeller). It is a slotted 12-pole motor, and draws 14 A of current at full load. It uses an electronic
speed controller that output a 3-phase voltage. Although commonly referred to as a DC motor, it really
is a miniature 3-phase AC permanent magnet synchronous motor, although induction types are sometimes
used in other applications.
Lithium-Polymer, abbreviated Li-Po or Li-Poly, evolved from lithium-ion (Li-Ion) chemistry. Li-Po
cells are rechargeable, and if treated carefully, can have a lifetime of many years and can be recharged
hundreds of times. The major distinction between Li-Ion and Li-Po is that the lithium-salt electrolyte
is not suspended in an organic solvent as it is in the Li-Ion cell. It uses a solid polymer composite
such as polyacrylonitrile (hence the "poly" for the electrolyte) as a physical separator, which reduces,
but does not eliminate, the opportunity for internal shorting that can cause fires and explosions. Lithium,
at atomic number 3, is a silvery white/gray metal that is highly reactive. Having an atomic number of
3 also accounts for its light weight. It is first in the series of alkali metals, and is the first solid
element. Lithium reacts with and can catch fire in water and water vapor in the presence of oxygen.
It also reacts with nitrogen, and since our air is 79% nitrogen, there is ample opportunity for a reaction
in everyday use if the packaging is breeched either by puncturing or overheating.
The video link above illustrates what can happen can happen to an overcharged Li-Poly battery pack.
Pictures have been published in modeling magazines of the burnt remains of airplanes and helicopters
whose owners did not properly recharge their batteries while the packs were still in the aircraft. The
recommended procedure for charging the Li-Po battery packs is to remove them from the aircraft and place
them in a fireproof container during the actual charging cycle. I use a clay flower pot as a container,
with a metal plate below to cover the water drain hole, and a metal plate above with a weight on top
of it. The charging cord rests in a notch that was made in the lip of the pot. Fortunately, after many
recharges, I have never had a fire incident.
Since lithium polymer (and lithium ion) are so sensitive to overcharging, the individual cells that
make up the battery pack are charged independently. With NiCad and NiMH packs, the standard charging
method is to apply a voltage and current across the series-connected cells so that the same current
passes through each cell. The voltage across the entire pack is the sum of the individual cells voltages,
but the cell voltages are not necessarily all equal. In fact, it is almost certain that they are not.
Since nickel-based cells are relatively tolerant of some overcharging, there is usually no danger. It
is not uncommon for one cell in a NiCad or NiMH battery pack to be dead, and yet the rest of the cells
charge normally. What that means is in the case of an 8-cell pack, with the nominal voltage of each
cell being 1.2 V (9.6 V total), if a charging voltage of 10.4 V is present, each cell would ideally
receive 10.4 / 8 V = 1.3 V. If one cell is dead, however, the voltage across each cell would be 10.4
/ 7 V = 1.5 V. That 15% increase can be handled by most NiCad and NiMH cells, but a 15% overvoltage
applied to a lithium cells would eventually result in a failure, and likely a fire. Li-Po batteries
are also intolerant of over discharging, and tend to die if discharged below around 2.5 V. In operation,
controller circuitry should prevent the cell voltage from dropping below 3.0 V. Cell temperature should
never exceed 90 °C in order to prevent the internal separator polymers from melting and allowing plate
shorting through physical contact.
 To the left is my Mini Pulse
XT aerobatic airplane. It uses the 450 motor and a 3-cell, 11.1 V, 2100 mAh, Li-Po battery. Futaba 4-channel
radio. To the left is my Mini Pulse
XT aerobatic airplane. It uses the 450 motor and a 3-cell, 11.1 V, 2100 mAh, Li-Po battery. Futaba 4-channel
radio.
Early lithium batteries had a rather high internal resistance, and had rather low discharge rates.
As with all technology that is doggedly pursued, significant improvements have been made to the point
that the contemporary Li-Po batteries may be substituted in most systems for the original NiCad or NiMH
batteries. Discharge rates of 20C or 25C are commonly available on the commercial market. In battery
discharge terminology, each "C" is a discharge current equivalent to the value of the energy capacity
of the cell (it is not the abbreviation for the Celsius degree unit). In the case of a 1,200
mAh rating, 1C is equal to a discharge current of 1,200 mA, or 1.2 amps. A 10C cell can deliver a continuous
current to a load of 10 x 1.2 A = 12 A during its discharge cycle. The E-flight 450 motor shown to the
right would require at least a 12C battery to deliver full rated power. This motor, by the way, is the
one I use in my electric-powered sailplane (2-meter wingspan) and in my 4-channel aerobatic airplane
(an E-flight Mini Pulse XT).
In my humble opinion, lithium batteries are grossly overpriced, given the huge production volumes
in effect. When you consider that almost every mobile electronic device and cordless power tool uses
these batteries, the cost should be a lot lower. Maybe it is partially product liability insurance that
manufacturers have to purchase that keeps the prices high, but I just paid $50 each for two 3-cell (11.1
V), 2,100 mAh packs. That is at least 2x to 3x what is should be costing at this point in the lithium
cell evolution.
 Here is the charging chemical reaction (from Quest Batteries): Here is the charging chemical reaction (from Quest Batteries):
LiCoO2 + 6C --> Li1-xCoO2 + LixC6
Here is the discharge chemical reaction (from Quest Batteries):
Li1-xCoO2 + LixC <----> Li1-x+dxCoO2
+ Lix-dxC
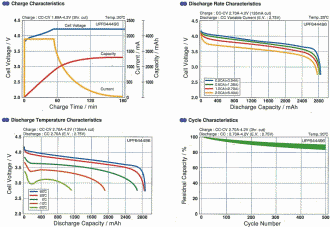
To the right are typical charge and discharge cycle charts for Li-Po batteries at room temperature
(20°C). These particular charts are based on
Sanyo data. Sanyo is currently the world's largest producer of lithium polymer batteries.
As you can see, the cell voltage is reasonably constant out to about the 20% capacity state, and then
falls off precipitously, similar to NiCad and NiMH cells.
 This
video of an overcharged lithium-polymer (Li-Po) battery will make you worry just a little about the
one sitting inside your cellphone, camera, or laptop computer. I use them in my R/C airplanes. Li-Po
cells are generally considered safer than lithium-ion (Li-Ion) cells, but they can - and do - still
catch fire in the wrong conditions. This
video of an overcharged lithium-polymer (Li-Po) battery will make you worry just a little about the
one sitting inside your cellphone, camera, or laptop computer. I use them in my R/C airplanes. Li-Po
cells are generally considered safer than lithium-ion (Li-Ion) cells, but they can - and do - still
catch fire in the wrong conditions.
There is a table of parameters for the most common battery types on the Battery Types & Specifications page here on RF Cafe.
Posted August 28, 2023
(updated from original post on 10/22/2008)
Related Pages on RF Cafe: -
Battery Drawings -
Battery Vendors - Li-Po
or Li-Poly Battery Characteristics -
Inside a 9-Volt Battery -
How Many AA Batteries Would to Take to Power a Human?
- Ray-O-Vac Ad, August 25, 1945, Saturday Evening Post
|












