[Go to TOC]
Module 1 - Introduction to Matter, Energy, and Direct Current
Pages i,
1-1,
1-11,
1-21,
1-31,
1-41,
1-51,
1-61,
2-1,
2-11,
2-21,
3-1,
3-11,
3-21,
3-31,
3-41,
3-51,
3-61,
3-71,
3-81,
3-91,
3-101,
3-111,
3-121, Appendix
I,
II,
III,
IV,
V,
Index
Chapter 2
Batteries
Learning Objectives
Upon completing this chapter, you will be able to:
1.
State the purpose a cell.
2. State the purpose the three parts a cell.
3. State the difference
between the two types cells.
4. Explain the chemical process that takes place in the primary and secondary
cells.
5. Recognize and define the terms electrochemical action, anode, cathode, and electrolyte.
6. State the causes polarization and local action and describe methods preventing these effects.
7. Identify the parts a dry cell.
8. Identify the various dry cells in use today and some
their capabilities and limitations.
9. Identify the four basic secondary cells, their construction,
capabilities, and limitations.
10. Define a battery, and identify the three ways combining cells to
form a battery.
11. Describe general maintenance procedures for batteries including the use the hydrometer,
battery capacity, and rating and battery charging.
12. Identify the five types battery charges.
13. Observe the safety precautions for working with and around batteries.
Introduction
The purpose of this chapter is to introduce and explain the basic theory and characteristics of batteries.
The batteries which are discussed and illustrated have been selected as representative many models and types which
are used in the Navy today. No attempt has been made to cover every type battery in use, however, after completing
this chapter you will have a good working knowledge the batteries which are in general use.
First, you will
learn about the building block all batteries, the CELL. The explanation will explore the physical makeup the cell
and the methods used to combine cells to provide useful voltage, current, and power. The chemistry the cell and
how chemical action is used to convert chemical energy to electrical energy are also discussed.
2-1
In addition, the care, maintenance, and operation batteries, as well as some the safety precautions
that should be followed while working with and around batteries are discussed.
Batteries are widely used
as sources direct-current electrical energy in automobiles, boats, aircraft, ships, portable electric/electronic
equipment, and lighting equipment. In some instances, they are used as the only source power; while in others, they
are used as a secondary or standby power source.
A battery consists a number cells assembled in a common
container and connected together to function as a source electrical power.
The CELL
A cell is a device that transforms chemical energy into electrical energy. The simplest cell, known as either
a galvanic or voltaic cell, is shown in figure 2-1. It consists a piece carbon (C) and a piece zinc (Zn) suspended
in a jar that contains a solution water (H2O) and sulfuric acid (H2SO 4) called the electrolyte.
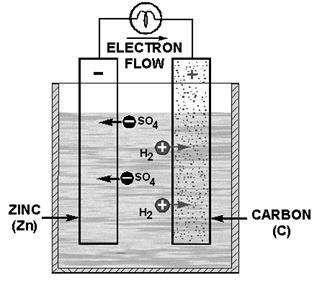
Figure 2-1. - Simple voltaic or galvanic cell.
The cell is the fundamental unit the battery. a simple cell consists two electrodes placed in a container
that holds the electrolyte.
In some cells the container acts as one the electrodes and, in this case, is
acted upon by the electrolyte. This will be covered in more detail later.
ELECTRODES
The electrodes are the conductors by which the current leaves or returns to the electrolyte. In the simple cell,
they are carbon and zinc strips that are placed in the electrolyte; while in the dry cell (fig.
2-2), they are
the carbon rod in the center and zinc container in which the cell is assembled.
2-2
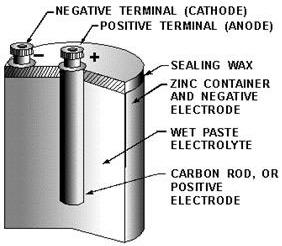
Figure 2-2. - Dry cell, cross-sectional view.
ELECTROLYTE
The electrolyte is the solution that acts upon the electrodes. The electrolyte,
which provides a path for electron flow, may be a salt, an acid, or an alkaline solution. In the simple galvanic
cell, the electrolyte is in a liquid form. In the dry cell, the electrolyte is a paste.
CONTAINER
The container which may be constructed one many different materials provides a means holding (containing) the
electrolyte. The container is also used to mount the electrodes. In the voltaic cell the container must be constructed
a material that will not be acted upon by the electrolyte.
Qi. What is the purpose a cell?
Q2.
What are the three parts a cell?
Q3. What is the purpose each the three parts a cell?
PRIMARY CELL
A primary cell is one in which the chemical action eats away one the electrodes,
usually the negative electrode. When this happens, the electrode must be replaced or the cell must be discarded.
In the galvanic-type cell, the zinc electrode and the liquid electrolyte are usually replaced when this happens.
In the case the dry cell, it is usually cheaper to buy a new cell.
SECONDARY CELL
A secondary cell is one in which the electrodes and the electrolyte are altered by the chemical action that
takes place when the cell delivers current. These cells may be restored to their original condition by forcing an
electric current through them in the direction opposite to that discharge. The automobile storage battery is a common
example the secondary cell.
2-3
Q4. What are the two types cells?
Q5. What is the main difference between the two
types cells?
ELECTROCHEMICAL ACTION
If a load (a device that consumes electrical power) is connected externally to the electrodes a cell, electrons
will flow under the influence a difference in potential across the electrodes from the CATHODE (negative electrode),
through the external conductor to the ANODE (positive electrode).
A cell is a device in which chemical energy
is converted to electrical energy. This process is called ELECTROCHEMICAL action.
The voltage across the
electrodes depends upon the materials from which the electrodes are made and the composition the electrolyte. The
current that a cell delivers depends upon the resistance the entire circuit, including that the cell itself. The
internal resistance the cell depends upon the size the electrodes, the distance between them in the electrolyte,
and the resistance the electrolyte. The larger the electrodes and the closer together they are in the electrolyte
(without touching), the lower the internal resistance the cell and the more current the cell is capable supplying
to the load.
Q6. What is electrochemical action?
Q7. What is another name for the (a)
positive electrode, and the (b) negative electrode?
PRIMARY CELL CHEMIsTRY
When a current flows through a primary cell having carbon and zinc electrodes and a diluted solution sulfuric
acid and water (combined to form the electrolyte), the following chemical reaction takes place.
The current
flow through the load is the movement electrons from the negative electrode the cell (zinc) and to the positive
electrode (carbon). This causes fewer electrons in the zinc and an excess electrons in the carbon. Figure 2-1 shows
the hydrogen ions (H2) from the sulfuric acid being attracted to the carbon electrode. Since the hydrogen ions are
positively charged, they are attracted to the negative charge on the carbon electrode. This negative charge is caused
by the excess electrons. The zinc electrode has a positive charge because it has lost electrons to the carbon electrode.
This positive charge attracts the negative ions (S04) from the sulfuric acid. The negative ions combine with the
zinc to form zinc sulfate. This action causes the zinc electrode to be eaten away. Zinc sulfate is a grayish-white
substance that is sometimes seen on the battery post an automobile battery.
The process the zinc being eaten
away and the sulfuric acid changing to hydrogen and zinc sulfate is the cause the cell discharging. When the zinc
is used up, the voltage the cell is reduced to zero.
In figure 2-1 you will notice that the zinc electrode
is labeled negative and the carbon electrode is labeled positive. This represents the current flow outside the cell
from negative to positive.
The zinc combines with the sulfuric acid to form zinc sulfate and hydrogen. The
zinc sulfate dissolves in the electrolyte (sulfuric acid and water) and the hydrogen appears as gas bubbles around
the carbon electrode. As current continues to flow, the zinc gradually dissolves and the solution changes to zinc
sulfate and water. The carbon electrode does not enter into the chemical changes taking place, but simply provides
a return path for the current.
2-4
Q8. In the primary cell, why are negative ions attracted to the negative terminal the cell?
Q9. How do electrons get from the negative electrode to the positive electrode?
Q1O. What causes the negative electrode to be eaten away?
SECONDARY CELL CHEMIsTRY
As stated before, the differences between primary and secondary cells are, the secondary cell can be recharged
and the electrodes are made different materials. The secondary cell shown in figure 2-3 uses sponge lead as the
cathode and lead peroxide as the anode. This is the lead-acid type cell and will be used to explain the general
chemistry the secondary cell. Later in the chapter when other types secondary cells are discussed, you will see
that the materials which make up the parts a cell are different, but that the chemical action is essentially the
same.
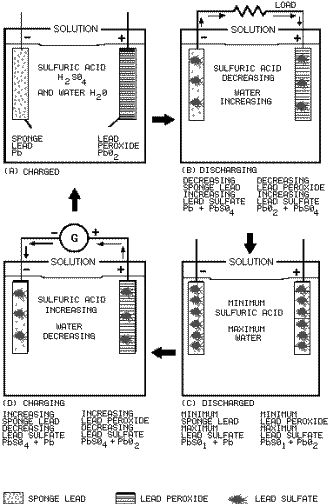
Figure 2-3. - Secondary cell.
2-5
Figure 2-3 view a shows a lead-acid secondary cell that is fully charged. The cathode is pure sponge
lead, the anode is pure lead peroxide, and the electrolyte is a mixture sulfuric acid and water.
Figure 2-3
view B shows the secondary cell discharging. a load is connected between the cathode and anode; current flows negative
to positive as shown. This current flow creates the same process as was explained for the primary cell with the
following exceptions.
In the primary cell the zinc cathode was eaten away by the sulfuric acid. In the secondary
cell the sponge-like construction the cathode retains the lead sulfate formed by the chemical action the sulfuric
acid and the lead. In the primary cell the carbon anode was not chemically acted upon by the sulfuric acid. In the
secondary cell the lead peroxide anode is chemically changed to lead sulfate by the sulfuric acid.
When the
cell is fully discharged it will be as shown in figure 2-3 view C. The anode and cathode retain some lead peroxide
and sponge lead but the amounts lead sulfate in each is maximum. The electrolyte has a minimum amount sulfuric acid.
With this condition no further chemical action can take place within the cell.
As you know, the secondary
cell can be recharged. Recharging is the process reversing the chemical action that occurs as the cell discharges.
To recharge the cell, a voltage source, such as a generator, is connected as shown in figure 2-3 view D. The negative
terminal the voltage source is connected to the cathode the cell and the positive terminal the voltage source is
connected to the anode the cell. With this arrangement the lead sulfate is chemically changed back to sponge lead
in the cathode, lead peroxide in the anode, and sulfuric acid in the electrolyte. After all the lead sulfate is
chemically changed, the cell is fully charged as shown in figure 2-3 view A. Once the cell has been charged, the
discharge-charge cycle may be repeated.
Q11. Refer to figure 2-3(B). Why is the sulfuric acid decreasing?
Q12. Refer to figure 2-3(D). How is it possible for the sulfuric acid to be increasing?
Q13.
Refer to figure 2-3(D). When all the lead sulfate has been converted, what is the condition the cell?
POLARIZATION of the CELL
The chemical action that occurs in the cell while the current is
flowing causes hydrogen bubbles to form on the surface the anode. This action is called POLARIZATION. Some hydrogen
bubbles rise to the surface the electrolyte and escape into the air, some remain on the surface the anode. If enough
bubbles remain around the anode, the bubbles form a barrier that increases internal resistance. When the internal
resistance the cell increases, the output current is decreased and the voltage the cell also decreases.
A
cell that is heavily polarized has no useful output. There are several methods to prevent polarization or to depolarize
the cell.
One method uses a vent on the cell to permit the hydrogen to escape into the air. a disadvantage
this method is that hydrogen is not available to reform into the electrolyte during recharging. This problem is
solved by adding water to the electrolyte, such as in an automobile battery. a second method is to use material
that is rich in oxygen, such as manganese dioxide, which supplies free oxygen to combine with the hydrogen and form
water.
2-6
A third method is to use a material that will absorb the hydrogen, such as calcium. The calcium releases
hydrogen during the charging process. All three methods remove enough hydrogen so that the cell is practically free
from polarization.
LOCAL ACTION
When the external circuit is removed, the current
ceases to flow, and, theoretically, all chemical action within the cell stops. However, commercial zinc contains
many impurities, such as iron, carbon, lead, and arsenic. These impurities form many small electrical cells within
the zinc electrode in which current flows between the zinc and its impurities. Thus, the chemical action continues
even though the cell itself is not connected to a load.
Local action may be prevented by using pure zinc
(which is not practical), by coating the zinc with mercury, or by adding a small percentage mercury to the zinc
during the manufacturing process. The treatment the zinc with mercury is called amalgamating (mixing) the zinc.
Since mercury is manytimes heavier than an equal volume water, small particles impurities weighing less than mercury
will float to the surface the mercury. The removal these impurities from the zinc prevents local action. The mercury
is not readily acted upon by the acid. When the cell is delivering current to a load, the mercury continues to act
on the impurities in the zinc. This causes the impurities to leave the surface the zinc electrode and float to the
surface the mercury. This process greatly increases the storage life the cell.
Q14. Describe three
ways to prevent polarization. Q15. Describe local action.
TYPES of CELLS
The development new and different types cells in the past decade has been so rapid that it is virtually
impossible to have a complete knowledge all the various types. a few recent developments are the silver-zinc, nickel-zinc,
nickel-cadmium, silver-cadmium, organic and inorganic lithium, and mercury cells.
PRIMARY DRY CELL
The dry cell is the most popular type primary cell. It is ideal for simple applications where an inexpensive
and noncritical source electricity is all that is needed.
The dry cell is not actually dry. The electrolyte
is not in a liquid state, but is a moist paste. If it should become totally dry, it would no longer be able to transform
chemical energy to electrical energy.
Construction of a Dry Cell
The construction
a common type dry cell is shown in figure 2-4. These dry cells are also referred to as Leclanche' cells. The internal
parts the cell are located in a cylindrical zinc container. This zinc container serves as the negative electrode
(cathode) the cell. The container is lined with a nonconducting material, such as blotting paper, to separate the
zinc from the paste. a carbon electrode is located in the center, and it serves as the positive terminal (anode)
the cell. The paste is a mixture several substances such as ammonium chloride, powdered coke, ground carbon, manganese
dioxide, zinc chloride, graphite, and water.
2-7
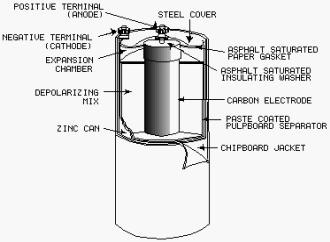
Figure 2-4. - Cutaway view the general-purpose dry cell.
This paste, which is packed in the space between the anode and the blotting paper, also serves to hold the
anode rigid in the center the cell. When the paste is packed in the cell, a small space is left at the top for expansion
the electrolytic paste caused by the depolarization action. The cell is then sealed with a cardboard or plastic
seal.
Since the zinc container is the cathode, it must be protected with some insulating material to be electrically
isolated. Therefore, it is common practice for the manufacturer to enclose the cells in cardboard and metal containers.
The dry cell (fig. 2-4) is basically the same as the simple voltaic cell (wet cell) described earlier, as far
as its internal chemical action is concerned. The action the water and the ammonium chloride in the paste, together
with the zinc and carbon electrodes, produces the voltage the cell. Manganese dioxide is added to reduce polarization
when current flows and zinc chloride reduces local action when the cell is not being used.
A cell that is
not being used (sitting on the shelf) will gradually deteriorate because slow internal chemical changes (local action).
This deterioration is usually very slow if cells are properly stored. If unused cells are stored in a cool place,
their shelf life will be greatly preserved. Therefore, to minimize deterioration, they should be stored in refrigerated
spaces.
The blotting paper (paste-coated pulpboard separator) serves two purposes - (1) it keeps the paste
from making actual contact with the zinc container and (2) it permits the electrolyte from the paste to filter through
to the zinc slowly. The cell is sealed at the top to keep air from entering and drying the electrolyte. Care should
be taken to prevent breaking this seal.
Q16. What serves as the cathode a dry cell?
Q17.
Why is a dry cell called a DRY cell?
Q18. What does the term "shelf life" mean?
2-8
Mercuric-Oxide Zinc Cell
The mercuric-oxide zinc cell (mercury cell) is a primary
cell that was developed during World War II. Two important assets the mercury cell are its ability to produce current
for a long period time and a long shelf life when compared to the dry cell shown in figure 2-4. The mercury cell
also has a very stable output voltage and is a power source that can be made in a small physical size.
With
the birth the space program and the development small transceivers and miniaturized equipment, a power source small
size was needed. Such equipment requires a small cell which is capable delivering maximum electrical energy at a
constant discharge voltage. The mercury cell, which is one the smallest cells, meets these requirements.
Present mercury cells are manufactured in three basic types as shown in figure 2-5. The wound- anode type, shown
in figure 2-5 view A, has an anode composed a corrugated zinc strip with a paper absorbent. The zinc is mixed with
mercury, and the paper is soaked in the electrolyte which causes it to swell and press against the zinc and make
positive contact. This process ensures that the electrolyte makes contact with the anode.
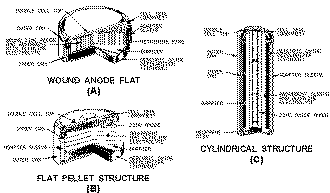
Figure 2-5. - Mercury cells.
In the pressed-powder cells, shown in figure 2-5 views B and C, the zinc powder for the anode is mixed prior
to being pressed into shape. The absorbent shown in the figure is paper soaked in the electrolyte. The space between
the inner and outer containers provides passage for any gas generated by an improper chemical balance or impurities
present within the cell.
If the anode and cathode a cell are connected together without a load, a Short Circuit
condition exists. Short circuits (shorts) can be very dangerous. They cause excessive heat, pressure, and current
flow which may cause serious damage to the cell or be a safety hazard to personnel.
2-9
Warning
Do not short the mercury cell. Shorted mercury cells have exploded with considerable force.
Other Types of Cells
There are many different types primary cells. Because such factors
as cost, size, ease replacement, and voltage or current needs, many types primary cells have been developed. The
following is a brief description some the primary cells in use today.
The Manganese Dioxide-Alkaline-Zinc
Cell is similar to the zinc-carbon cell except for the electrolyte used. This type cell offers better voltage stability
and longer life than the zinc-carbon type. It also has a longer shelf life and can operate over a wide temperature
range. The manganese dioxide- alkaline-zinc cell has a voltage 1.5 volts and is available in a wide range sizes.
This cell is commonly referred to as the alkaline cell.
The Magnesium-Manganese Dioxide Cell uses magnesium
as the anode material. This allows a higher output capacity over an extended period time compared to the zinc-carbon
cell. This cell produces a voltage approximately 2 volts. The disadvantage this type cell is the production hydrogen
during its operation.
The Lithium-Organic Cell and the Lithium-Inorganic Cell are recent developments a new
line high-energy cells. The main advantages these types cells are very high power, operation over a wide temperature
range, they are lighter than most cells, and have a remarkably long shelf life up to 20
years.
Caution
Lithium cells contain toxic materials under pressure. Do not puncture, recharge, short-circuit, expose to
excessively high temperatures, or incinerate. use these batteries/cells only in approved equipment. Do not throw
in trash.
Q19. Why should a mercury cell NOT be shorted?
Q20. What factors should be considered
when selecting a primary cell for a power source?
SECONDARY WET CELLS
Secondary cells
are sometimes known as wet cells. There are four basic type wet cells, the lead- acid, nickel-cadmium, silver-zinc,
and silver-cadmium.
Lead-Acid Cell
The lead-acid cell is the most widely used secondary
cell. The previous explanation the secondary cell describes exactly the manner in which the lead-acid cell provides
electrical power. The discharging and charging action presented in electrochemical action describes the lead-acid
cell.
You should recall that the lead-acid cell has an anode lead peroxide, a cathode sponge lead,
and the electrolyte is sulfuric acid and water
2-10
| - |
Matter, Energy,
and Direct Current |
| - |
Alternating Current and Transformers |
| - |
Circuit Protection, Control, and Measurement |
| - |
Electrical Conductors, Wiring Techniques,
and Schematic Reading |
| - |
Generators and Motors |
| - |
Electronic Emission, Tubes, and Power Supplies |
| - |
Solid-State Devices and Power Supplies |
| - |
Amplifiers |
| - |
Wave-Generation and Wave-Shaping Circuits |
| - |
Wave Propagation, Transmission Lines, and
Antennas |
| - |
Microwave Principles |
| - |
Modulation Principles |
| - |
Introduction to Number Systems and Logic Circuits |
| - |
- Introduction to Microelectronics |
| - |
Principles of Synchros, Servos, and Gyros |
| - |
Introduction to Test Equipment |
| - |
Radio-Frequency Communications Principles |
| - |
Radar Principles |
| - |
The Technician's Handbook, Master Glossary |
| - |
Test Methods and Practices |
| - |
Introduction to Digital Computers |
| - |
Magnetic Recording |
| - |
Introduction to Fiber Optics |
| Note: Navy Electricity and Electronics Training
Series (NEETS) content is U.S. Navy property in the public domain. |