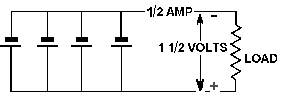[Go to TOC]
Module 1 - Introduction to Matter, Energy, and Direct Current
Pages i,
1-1,
1-11,
1-21,
1-31,
1-41,
1-51,
1-61,
2-1,
2-11,
2-21,
3-1,
3-11,
3-21,
3-31,
3-41,
3-51,
3-61,
3-71,
3-81,
3-91,
3-101,
3-111,
3-121, Appendix
I,
II,
III,
IV,
V,
Index
Several new terms were
introduced in this chapter. The following is a summary the chapter on batteries.
A CELL
is a device that transforms chemical energy into electrical energy. The cell has three parts; the electrodes, the
electrolyte, and the container. There are two basic cells: primary and secondary.
The ELECTRODES are the current conductors the cell.
The ELECTROLYTE
is the solution that acts upon the electrodes.
The CONTAINER holds the electrolyte and provides
a means mounting the electrodes.
The PRIMARY CELL is a cell in which the chemical action
finally destroys one the electrodes, usually the negative. The primary cell cannot be recharged.
The
SECONDARY CELL is a cell in which the chemical action alters the electrodes and electrolyte. The
electrodes and electrolyte can be restored to their original condition by recharging the cell.
2-21
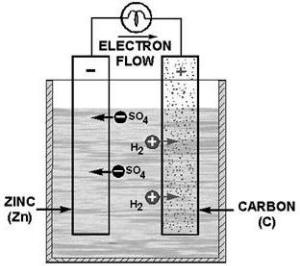
ELECTROCHEMICAL ACTION is the process converting chemical energy into electrical energy.
The ANODE is the positive electrode a cell.
The CATHODE is the negative
electrode a cell.
PRIMARY CELL CHEMIsTRY is the process in which electrons leaving the cathode
to the load cause a positive charge which attracts negative ions from the electrolyte. The negative ions combine
with the material the cathode and form a substance such as lead-sulfate. Electrons from the load to the anode create
a negative charge which attracts positive ions (hydrogen) from the electrolyte.
2-22
SECONDARY CELL CHEMIsTRY is the process in which the electrolyte acts upon and chemically
changes both electrodes. This process also depletes the amount active material in the electrolyte. a charging current
applied to the cell reverses the process and restores the cell to its original condition.
POLARIZATION
is the effect hydrogen surrounding the anode a cell which increases the internal resistance the cell. Polarization
can be prevented by venting the cell, adding a material rich in oxygen, or adding a material that will absorb hydrogen.
LOCAL ACTION is the continuation current flow within the cell when there is no external load.
It is caused by impurities in the electrode and can be prevented by the use mercury amalgamated with the material
the electrode.
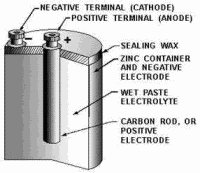
DRY CELL is the type commonly referred to as the "flashlight battery." Since
the electrolyte is not in liquid form, but is a paste, the term dry cell is used. In most dry cells the case is
the cathode.
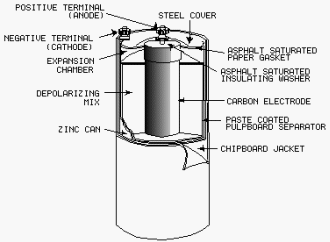
2-23
SHELF LIFE is the period the cell may be stored and still be usable.
MERCURY CELLS should never be shorted because the danger explosion.

DRY CELLS are many types, each having advantages and disadvantages. The type selected for use depends on such factors as cost, size, ease replacement, and voltage or current needs.
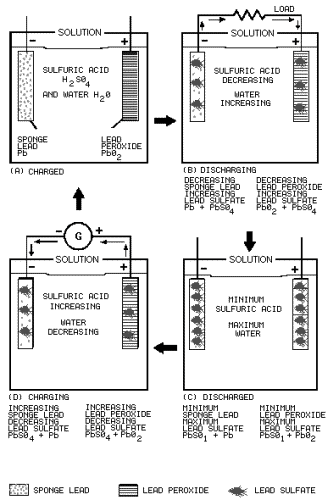
The LEAD-ACID
CELL is the most widely used secondary cell. The lead-acid cell produces electricity by electrochemical
action. The anode is lead peroxide, the cathode is sponge lead, and the electrolyte is sulfuric acid and water.

The NICKEL-CADMIUM CELL, commonly called the NICAD, has the following advantages over the
lead-acid cell; charges in a shorter period time, delivers a larger amount power, stays idle longer, and can be
charged and discharged many times. The anode is nickel hydroxide, the cathode is cadmium hydroxide, and the electrolyte
is potassium hydroxide and water.
2-24
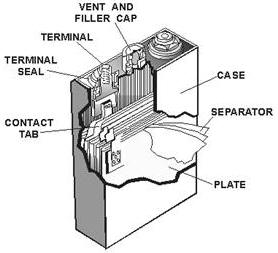
The SILVER-ZINC CELL is used mostly for emergency equipment. It is light, small, and has
a large power capacity for its size. The anode is silver oxide, the cathode is zinc, and the electrolyte is potassium
hydroxide and water.
The SILVER-CADMIUM CELL combines the better features the nickel-cadmium
and silver- zinc cells. The anode is silver-oxide, the cathode is cadmium hydroxide, and the electrolyte is potassium
hydroxide.
A BATTERY is a voltage source in a single container made from one or more cells.
The cells can be combined in series, parallel, or series-parallel.

2-25
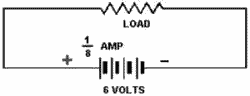
Series CONNECTED CELLS provide a higher voltage than a single cell, with no increase
in current.
PARALLEL CONNECTED CELLS provide a higher current than a single cell, with no increase
in voltage.
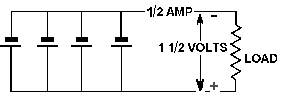
Series-PARALLEL CONNECTED CELLS provide a higher voltage and a higher current than
a single cell.
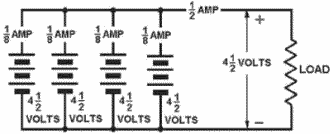
TYPES of BATTERIES can be determined from nameplate data.
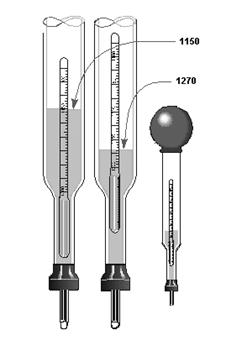
HYDROMETER
provides the means to check the specific gravity the electrolyte.
2-26
Safety PRECautionS should always be followed when working with or around batteries.
CAPACITY is an indication the current-supplying capability the battery for a specific period
time; e.g., 400 ampere-hour.
RATING is the capacity the battery for a specific rate discharge.
In most batteries the rating is given for a 20 hour discharge cycle; e.g., 20 amperes for 20 hours.
BATTERY CHARGE is the process reversing the current flow through the battery to restore the battery
to its original condition. The addition active ingredient to the electrolyte will not recharge the battery. There
are five types charges:
1. Initial charge
2. Normal charge
3.
Equalizing charge
4. Floating charge
5. Fast charge
GASSING
is the production hydrogen gas caused by a portion the charge current breaking down the water in the electrolyte.
Steady gassing is normal during the charging process. Violent gassing indicates that the charge rate is too high.
2-27
Answers to Questions Q1. Through Q34.
A1. a cell is a device that converts chemical energy to electrical energy.
A2.
The electrodes, the electrolyte, and the container.
A3. The electrodes are the current conductors the
cell. The electrolyte is the solution that acts upon the electrodes. The container holds the electrolyte and provides
a means mounting the electrodes.
A4. Primary and secondary.
A5. The secondary cell can
be restored to its original condition by an electric current. The primary cell cannot.
A6. The process
converting chemical energy into electrical energy.
A7. (a) The anode, (b) the cathode.
A8.
The positive charge caused by electrons leaving the negative electrode attracts the negative ions.
A9.
By current flow through the load.
A10. The chemical action between the negative electrode and the electrolyte.
A11. The sulfuric acid is chemically acting upon the anode and cathode which creates a current flow through
the load.
A12. The charging currents causes the lead sulfate in the anode and cathode to be changed
back to lead peroxide, sponge lead, and sulfuric acid.
A13. Fully charged.
A14. Vent the
cell, add a material rich in oxygen, and use a material that will absorb hydrogen. A15. Current flow in a
cell with no external load.
A16. The zinc container.
A17. The electrolyte is not a liquid
but is in the form a paste.
A18. The period that a cell can be stored and still be useable.
A19. The danger explosion.
A20. Cost, size, ease replacement, and voltage or current needs.
A21.Lead-acid, nickel-cadmium (NICAD), silver-zinc, and silver-cadmium.
A22. Can be charged in a shorter
time, can deliver a larger amount power, and stays idle longer.
A23. Silver-zinc cell.
A24.
Silver-cadmium, silver-zinc, and nickel-cadmium.
A25. a voltage source in a single container made from
one or more cells.
2-28
A26. Series, to increase voltage but not current. Parallel, to increase current but not voltage.
Series- Parallel, to increase both current and voltage.
A27. The cells in the NiCad battery can be
replaced. A28.By looking at the nameplate data.
A29. To measure the amount active ingredient in the
electrolyte.
A30. Electrolyte B. It is heavier per unit volume.
A31. At all times.
A32. Forty hours.
A33. No, a current must be passed through the battery.
A34.
Reduce the charging rate.
2-29
| - |
Matter, Energy,
and Direct Current |
| - |
Alternating Current and Transformers |
| - |
Circuit Protection, Control, and Measurement |
| - |
Electrical Conductors, Wiring Techniques,
and Schematic Reading |
| - |
Generators and Motors |
| - |
Electronic Emission, Tubes, and Power Supplies |
| - |
Solid-State Devices and Power Supplies |
| - |
Amplifiers |
| - |
Wave-Generation and Wave-Shaping Circuits |
| - |
Wave Propagation, Transmission Lines, and
Antennas |
| - |
Microwave Principles |
| - |
Modulation Principles |
| - |
Introduction to Number Systems and Logic Circuits |
| - |
- Introduction to Microelectronics |
| - |
Principles of Synchros, Servos, and Gyros |
| - |
Introduction to Test Equipment |
| - |
Radio-Frequency Communications Principles |
| - |
Radar Principles |
| - |
The Technician's Handbook, Master Glossary |
| - |
Test Methods and Practices |
| - |
Introduction to Digital Computers |
| - |
Magnetic Recording |
| - |
Introduction to Fiber Optics |
| Note: Navy Electricity and Electronics Training
Series (NEETS) content is U.S. Navy property in the public domain. |